Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway
- PMID: 15385484
- PMCID: PMC517603
- DOI: 10.1128/IAI.72.10.5832-5839.2004
Bacteroides fragilis enterotoxin induces intestinal epithelial cell secretion of interleukin-8 through mitogen-activated protein kinases and a tyrosine kinase-regulated nuclear factor-kappaB pathway
Abstract
Enterotoxigenic Bacteroides fragilis (ETBF) secretes a 20-kDa metalloprotease toxin termed B. fragilis toxin (BFT). ETBF disease in animals is associated with an acute inflammatory response in the intestinal mucosa, and lethal hemorrhagic colitis may occur in rabbits. In this study, we confirmed recent reports (J. M. Kim, Y. K. Oh, Y. J. Kim, H. B. Oh, and Y. J. Cho, Clin. Exp. Immunol. 123:421-427, 2001; L. Sanfilippo, C. K. Li, R. Seth, T. J. Balwin, M. J. Menozzi, and Y. R. Mahida, Clin. Exp. Immunol. 119:456-463, 2000) that purified BFT stimulates interleukin-8 (IL-8) secretion by human intestinal epithelial cells (HT29/C1 cells) and demonstrate that stimulation of IL-8 production is dependent on biologically active BFT and independent of serum. Induction of IL-8 mRNA expression occurs rapidly and ceases by 6 h after BFT treatment, whereas IL-8 secretion continues to increase for at least 18 h. Our data suggest that BFT-stimulated IL-8 secretion involves tyrosine kinase-dependent activation of nuclear factor-kappaB (NF-kappaB) as well as activation of the mitogen-activated protein kinases (MAPKs), p38 and extracellular signal-related kinase. Simultaneous activation of NF-kappaB and MAPKs appears necessary for secretion of IL-8 by HT29/C1 cells treated with BFT.
Figures
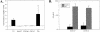


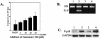

References
-
- Banks, C., A. Bateman, R. Payne, P. Johnson, and N. Sheron. 2003. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J. Pathol. 199:28-35. - PubMed
-
- Blaecke, A., Y. Delneste, N. Herbault, P. Jeannin, J. Y. Bonnefoy, A. Beck, and J. P. Aubry. 2002. Measurement of nuclear factor-kappa B translocation on lipopolysaccharide-activated human dendritic cells by confocal microscopy and flow cytometry. Cytometry 48:71-79. - PubMed
-
- Craig, R., A. Larkin, A. M. Mingo, D. J. Thuerauf, C. Andrews, P. M. McDonough, and C. C. Glembotski. 2000. p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J. Biol. Chem. 275:23814-23824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

