A new cruzipain-mediated pathway of human cell invasion by Trypanosoma cruzi requires trypomastigote membranes
- PMID: 15385491
- PMCID: PMC517595
- DOI: 10.1128/IAI.72.10.5892-5902.2004
A new cruzipain-mediated pathway of human cell invasion by Trypanosoma cruzi requires trypomastigote membranes
Abstract
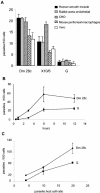
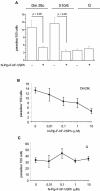
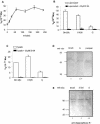
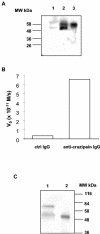
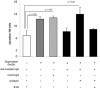
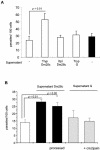
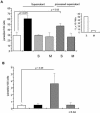
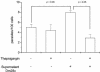
The intracellular protozoan Trypanosoma cruzi causes Chagas' disease, a chronic illness associated with cardiomyopathy and digestive disorders. This pathogen invades mammalian cells by signaling them through multiple transduction pathways. We previously showed that cruzipain, the main cysteine protease of T. cruzi, promotes host cell invasion by activating kinin receptors. Here, we report a cruzipain-mediated invasion route that is not blocked by kinin receptor antagonists. By testing different strains of T. cruzi, we observed a correlation between the level of cruzipain secreted by trypomastigotes and the capacity of the pathogen to invade host cells. Consistent with a role for cruzipain, the cysteine protease inhibitor N-methylpiperazine-urea-Phe-homophenylalanine-vinylsulfone-benzene impaired the invasion of human smooth muscle cells by strains Dm28c and X10/6 but not by the G isolate. Cruzipain-rich supernatants of Dm28c trypomastigotes enhanced the infectivity of isolate G parasites twofold, an effect which was abolished by the cysteine protease inhibitor l-trans-epoxysuccinyl-leucylamido-(4-guanidino)butane and by thapsigargin, a drug that induces depletion of the intracellular Ca(2+) stores. The enhancement due to Dm28 supernatants was abolished upon cruzipain immunodepletion, and the activity was restored by purified cruzipain. In contrast, supernatants from isolate G trypomastigotes (with low levels of cruzipain) or supernatants from Dm28c epimastigotes or purified cruzipain alone did not enhance parasite invasion, indicating that the protease is required but not sufficient to engage this invasion pathway. We provide evidence that activation of this pathway requires cruzipain-mediated processing of a trypomastigote molecule associated with parasite-shed membranes. Our results couple cruzipain to host cell invasion through a kinin-independent route and further suggest that high-level cruzipain expression may contribute to parasite infectivity.
Figures

Similar articles
-
Host cell invasion by Trypanosoma cruzi is potentiated by activation of bradykinin B(2) receptors.J Exp Med. 2000 Nov 6;192(9):1289-300. doi: 10.1084/jem.192.9.1289. J Exp Med. 2000. PMID: 11067878 Free PMC article.
-
Cruzipain Activates Latent TGF-β from Host Cells during T. cruzi Invasion.PLoS One. 2015 May 4;10(5):e0124832. doi: 10.1371/journal.pone.0124832. eCollection 2015. PLoS One. 2015. PMID: 25938232 Free PMC article.
-
Cruzipain promotes Trypanosoma cruzi adhesion to Rhodnius prolixus midgut.PLoS Negl Trop Dis. 2012;6(12):e1958. doi: 10.1371/journal.pntd.0001958. Epub 2012 Dec 13. PLoS Negl Trop Dis. 2012. PMID: 23272264 Free PMC article.
-
Roles of naturally occurring protease inhibitors in the modulation of host cell signaling and cellular invasion by Trypanosoma cruzi.Subcell Biochem. 2008;47:140-54. doi: 10.1007/978-0-387-78267-6_11. Subcell Biochem. 2008. PMID: 18512348 Review.
-
Trypanosoma cruzi infection by oral route: how the interplay between parasite and host components modulates infectivity.Parasitol Int. 2008 Jun;57(2):105-9. doi: 10.1016/j.parint.2007.12.008. Epub 2007 Dec 23. Parasitol Int. 2008. PMID: 18234547 Review.
Cited by
-
Targeting Cysteine Proteases and their Inhibitors to Combat Trypanosomiasis.Curr Med Chem. 2024;31(16):2135-2169. doi: 10.2174/0929867330666230619160509. Curr Med Chem. 2024. PMID: 37340748 Review.
-
Reversible cysteine protease inhibitors show promise for a Chagas disease cure.Antimicrob Agents Chemother. 2014;58(2):1167-78. doi: 10.1128/AAC.01855-13. Epub 2013 Dec 9. Antimicrob Agents Chemother. 2014. PMID: 24323474 Free PMC article.
-
Proteomic analysis reveals different composition of extracellular vesicles released by two Trypanosoma cruzi strains associated with their distinct interaction with host cells.J Extracell Vesicles. 2018 Apr 17;7(1):1463779. doi: 10.1080/20013078.2018.1463779. eCollection 2018. J Extracell Vesicles. 2018. PMID: 29696081 Free PMC article.
-
Blood-brain barrier traversal by African trypanosomes requires calcium signaling induced by parasite cysteine protease.J Clin Invest. 2006 Oct;116(10):2739-47. doi: 10.1172/JCI27798. Epub 2006 Sep 21. J Clin Invest. 2006. PMID: 16998589 Free PMC article.
-
Specific Endocytosis Blockade of Trypanosoma cruzi Exposed to a Poly-LAcNAc Binding Lectin Suggests that Lectin-Sugar Interactions Participate to Receptor-Mediated Endocytosis.PLoS One. 2016 Sep 29;11(9):e0163302. doi: 10.1371/journal.pone.0163302. eCollection 2016. PLoS One. 2016. PMID: 27685262 Free PMC article.
References
-
- Burleigh, B. A., and A. M. Woolsey. 2002. Cell signaling and Trypanosoma cruzi invasion. Cell. Microbiol. 4:701-711. - PubMed
-
- Campetella, O., J. Henriksson, L. Aslund, A. C. C. Frasch, U. Pettterson, and J. J. Cazzulo. 1992. The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is encoded by multiple polymorphic tandemly organized genes located on different chromosomes. Mol. Biochem. Parasitol. 50:225-234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

