Porphyromonas gingivalis strain-dependent activation of human endothelial cells
- PMID: 15385493
- PMCID: PMC517532
- DOI: 10.1128/IAI.72.10.5910-5918.2004
Porphyromonas gingivalis strain-dependent activation of human endothelial cells
Abstract
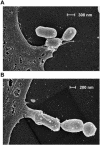
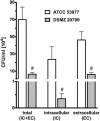
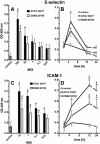
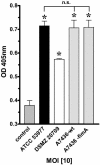
Porphyromonas gingivalis is an important bacterium involved in periodontal diseases. Colonization by periodontopathogens has been associated with severe local inflammatory reactions in the connective tissue. In this study we characterized P. gingivalis-mediated infection and activation of human umbilical vein endothelial cells by using two strains of different virulence capacities, strains ATCC 53977 and DSMZ 20709. Both strains were able to adhere to and infect endothelial cells with an infection rate of 0.48% for ATCC 53977 and 0.007% for DSMZ 20709. The triggering of two signal transduction pathways in P. gingivalis-infected endothelial cells was demonstrated for both strains, with a rapid increase of p38 mitogen-activated protein kinase phosphorylation and a more delayed degradation of IkappaBalpha, followed by nuclear translocation of NF-kappaB. In addition, both strains induced enhanced expression of endothelial adhesion molecules E-selectin and intracellular adhesion molecule 1 (ICAM-1). Target cell activation was independent of bacterial fimbriae expression since the fimA knockout strain A7436 DeltafimA induced the same level of ICAM-1 as the corresponding wild type (A7436-WT). Thus, two P. gingivalis strains, ATCC 53799 and DSMZ 20709, infect endothelial cells and trigger signaling cascades leading to endothelial activation, which in turn may result in or promote severe local and systemic inflammation.
Figures


Similar articles
-
Porphorymonas gingivalis induces intracellular adhesion molecule-1 expression in endothelial cells through the nuclear factor-kappaB pathway, but not through the p38 MAPK pathway.J Periodontal Res. 2011 Feb;46(1):31-8. doi: 10.1111/j.1600-0765.2010.01305.x. Epub 2010 Sep 3. J Periodontal Res. 2011. PMID: 20825555
-
Fimbria-dependent activation of pro-inflammatory molecules in Porphyromonas gingivalis infected human aortic endothelial cells.Cell Microbiol. 2006 May;8(5):738-57. doi: 10.1111/j.1462-5822.2005.00661.x. Cell Microbiol. 2006. PMID: 16611224
-
E-selectin expression induced by Porphyromonas gingivalis in human endothelial cells via nucleotide-binding oligomerization domain-like receptors and Toll-like receptors.Mol Oral Microbiol. 2015 Oct;30(5):399-410. doi: 10.1111/omi.12102. Epub 2015 Jun 24. Mol Oral Microbiol. 2015. PMID: 25939768
-
Porphyromonas gingivalis fimbriae-dependent interleukin-6 autocrine regulation by increase of gp130 in endothelial cells.J Periodontal Res. 2009 Aug;44(4):550-6. doi: 10.1111/j.1600-0765.2008.01150.x. Epub 2009 Mar 13. J Periodontal Res. 2009. PMID: 19438975
-
Paeonol suppresses intercellular adhesion molecule-1 expression in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells by blocking p38, ERK and nuclear factor-kappaB signaling pathways.Int Immunopharmacol. 2007 Mar;7(3):343-50. doi: 10.1016/j.intimp.2006.11.004. Epub 2006 Dec 14. Int Immunopharmacol. 2007. PMID: 17276892
Cited by
-
Pathogen-mediated proteolysis of the cell death regulator RIPK1 and the host defense modulator RIPK2 in human aortic endothelial cells.PLoS Pathog. 2012;8(6):e1002723. doi: 10.1371/journal.ppat.1002723. Epub 2012 Jun 7. PLoS Pathog. 2012. PMID: 22685397 Free PMC article.
-
Influence of the oscillation frequency of different side-to-side toothbrushes on noncontact biofilm removal.Clin Oral Investig. 2018 Jul;22(6):2141-2147. doi: 10.1007/s00784-017-2305-x. Epub 2018 Jan 22. Clin Oral Investig. 2018. PMID: 29356919
-
Plant-derived pectin nanocoatings to prevent inflammatory cellular response of osteoblasts following Porphyromonas gingivalis infection.Int J Nanomedicine. 2017 Jan 12;12:433-445. doi: 10.2147/IJN.S113740. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28138240 Free PMC article.
-
Alzheimer's Disease-Like Neurodegeneration in Porphyromonas gingivalis Infected Neurons with Persistent Expression of Active Gingipains.J Alzheimers Dis. 2020;75(4):1361-1376. doi: 10.3233/JAD-200393. J Alzheimers Dis. 2020. PMID: 32390638 Free PMC article.
-
Interrupting oral infection of Porphyromonas gingivalis with anti-FimA antibody attenuates bacterial dissemination to the arthritic joint and improves experimental arthritis.Exp Mol Med. 2018 Mar 23;50(3):e460. doi: 10.1038/emm.2017.301. Exp Mol Med. 2018. PMID: 29568073 Free PMC article.
References
-
- Amar, S., N. Gokce, S. Morgan, M. Loukideli, T. E. Van Dyke, and J. A. Vita. 2003. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler. Thromb. Vasc. Biol. 23:1245-1249. - PubMed
-
- Bonfanti, R., B. C. Furie, B. Furie, and D. D. Wagner. 1989. PADGEM (GMP140) is a component of Weibel-Palade bodies of human endothelial cells. Blood 73:1109-1112. - PubMed
-
- Burdon, K. L. 1928. Bacterium melaninogenicum from normal and pathogenic tissues. J. Infect. Dis. 42:161-171.
-
- Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. - PubMed
-
- Chen, C. C., and A. M. Manning. 1995. Transcriptional regulation of endothelial cell adhesion molecules: a dominant role for NF-kappa B. Agents Actions Suppl. 47:135-141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

