Enhanced lung injury and delayed clearance of Pneumocystis carinii in surfactant protein A-deficient mice: attenuation of cytokine responses and reactive oxygen-nitrogen species
- PMID: 15385504
- PMCID: PMC517574
- DOI: 10.1128/IAI.72.10.6002-6011.2004
Enhanced lung injury and delayed clearance of Pneumocystis carinii in surfactant protein A-deficient mice: attenuation of cytokine responses and reactive oxygen-nitrogen species
Abstract
Surfactant protein A (SP-A), a member of the collectin family, selectively binds to Pneumocystis carinii and mediates interactions between pathogen and host alveolar macrophages in vitro. To test the hypothesis that mice lacking SP-A have delayed clearance of Pneumocystis organisms and enhanced lung injury, wild-type C57BL/6 (WT) and SP-A-deficient mice (SP-A(-/-)) with or without selective CD4(+)-T-cell depletion were intratracheally inoculated with Pneumocystis organisms. Four weeks later, CD4-depleted SP-A-deficient mice had developed a more severe Pneumocystis infection than CD4-depleted WT (P. carinii pneumonia [PCP] scores of 3 versus 2, respectively). Whereas all non-CD4-depleted WT mice were free of PCP, intact SP-A(-/-) mice also had evidence of increased organism burden. Pneumocystis infection in SP-A-deficient mice was associated histologically with enhanced peribronchial and/or perivascular cellularity (score of 4 versus 2, SP-A(-/-) versus C57BL/6 mice, respectively) and a corresponding increase in bronchoalveolar lavage (BAL) cell counts. Increases in SP-D content, gamma interferon, interleukin-4, interleukin-5, and tumor necrosis factor alpha in BAL fluid occurred but were attenuated in PCP-infected SP-A(-/-) mice compared to WT mice. There were increases in total BAL NO levels in both infected groups, but nitrite levels were higher in SP-A(-/-) mice, indicating a reduction in production of higher oxides of nitrogen that was also reflected in lower levels of 3-nitrotyrosine staining in the SP-A(-/-) group. We conclude that despite increases in inflammatory cells, SP-A-deficient mice infected with P. carinii exhibit an enhanced susceptibility to the organism and attenuated production of proinflammatory cytokines and reactive oxygen-nitrogen species. These data support the concept that SP-A is a local effector molecule in the lung host defense against P. carinii in vivo.
Figures
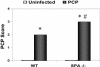

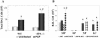

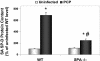

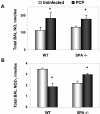
 , uninfected mice; ▪, P. carinii-infected mice. ✽, Significant difference from the corresponding uninfected level (P < 0.05); #, significant difference from the corresponding WT level (P < 0.05).
, uninfected mice; ▪, P. carinii-infected mice. ✽, Significant difference from the corresponding uninfected level (P < 0.05); #, significant difference from the corresponding WT level (P < 0.05).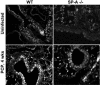
References
-
- Atochina, E. N., A. J. Gow, J. M. Beck, A. Haczku, A. Inch, H. Kadire, Y. Tomer, C. Davis, A. M. Preston, F. Poulain, S. Hawgood, and M. F. Beers. 2004. Surfactant protein D deficient mice exhibit delayed clearance of Pneumocystis lung infection with increased inflammation and altered nitric oxide metabolism. J. Infect. Dis. 189:1528-1539. - PubMed
-
- Atochina, E. N., J. M. Beck, S. T. Scanlon, A. M. Preston, and M. F. Beers. 2001. Pneumocystis carinii pneumonia alters expression and distribution of lung collectins SP-A and SP-D. J. Lab. Clin. Med. 137:429-439. - PubMed
-
- Atochina, E. N., M. F. Beers, S. T. Scanlon, A. M. Preston, and J. M. Beck. 2000. Pneumocystis carinii induces selective alterations in component expression and biophysical activity of lung surfactant. Am. J. Physiol. 278:L599-L609. - PubMed
-
- Atochina, E. N., M. F. Beers, S. Hawgood, F. Poulain, C. Davis, T. T. Fusaro, and A. J. Gow. 2003. Surfactant protein-D, a mediator of innate lung immunity, alters the products of nitric oxide metabolism. Am. J. Respir. Cell Mol. Biol. 30:271-279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

