Development and characterization of an in vivo central venous catheter Candida albicans biofilm model
- PMID: 15385506
- PMCID: PMC517581
- DOI: 10.1128/IAI.72.10.6023-6031.2004
Development and characterization of an in vivo central venous catheter Candida albicans biofilm model
Abstract
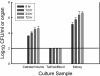
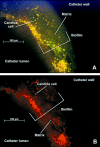
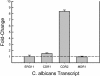
Biofilms represent a niche for microorganisms where they are protected from both the host immune system and antimicrobial therapies. Biofilm growth serves as an increasing source of clinical infections. Candida infections are difficult to manage due to their persistent nature and associated drug resistance. Observations made in biofilm research have generally been limited to in vitro models. Using a rat central venous catheter model, we characterized in vivo Candida albicans biofilm development. Time-course quantitative culture demonstrated a progressive increase in the burden of viable cells for the first 24 h of development. Fluorescence and scanning electron microscopy revealed a bilayered architecture. Adjacent to the catheter surface, yeast cells were densely embedded in an extracellular matrix. The layer adjacent to the catheter lumen was less dense. The outermost surface of the biofilm contained both yeast and hyphal forms, and the extracellular material in which they were embedded appeared fibrous. These architectural features were similar in many respects to those described for in vitro models. However, scanning electron microscopy also revealed host cells embedded within the biofilm matrix. Drug susceptibility was determined by using two assays and demonstrated a biofilm-associated drug resistance phenotype. The first assay demonstrated continued growth of cells in the presence of supra-MIC antifungal drug concentrations. The second assay demonstrated reduced susceptibility of biofilm-grown cells following removal from the biofilm structure. Lastly, the model provided sufficient nucleic material for study of differential gene expression associated with in vivo biofilm growth. Two fluconazole efflux pumps, CDR1 and CDR2, were upregulated in the in vivo biofilm-associated cells. Most importantly, the studies described provide a model for further investigation into the molecular mechanisms of C. albicans biofilm biology and drug resistance. In addition, the model provides a means to study novel drug therapies and device technologies targeted to the control of biofilm-associated infections.
Figures



References
-
- Anaissie, E. J., J. H. Rex, O. Uzun, and S. Vartivarian. 1998. Predictors of adverse outcome in cancer patients with candidemia. Am. J. Med. 104:238-245. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 2000. Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal drugs. J. Antimicrob. Chemother. 46:397-403. - PubMed
-
- Baillie, G. S., and L. J. Douglas. 1999. Role of dimorphism in the development of Candida albicans biofilms. J. Med. Microbiol. 48:671-679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

