The Streptococcus pyogenes capsule is required for adhesion of bacteria to virus-infected alveolar epithelial cells and lethal bacterial-viral superinfection
- PMID: 15385511
- PMCID: PMC517596
- DOI: 10.1128/IAI.72.10.6068-6075.2004
The Streptococcus pyogenes capsule is required for adhesion of bacteria to virus-infected alveolar epithelial cells and lethal bacterial-viral superinfection
Abstract
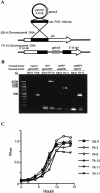
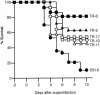
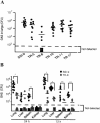
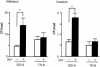
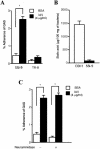
An apparent worldwide resurgence of invasive group A Streptococcus (GAS) infections remains unexplained. However, we recently demonstrated in mice that when an otherwise nonlethal intranasal GAS infection is preceded by a nonlethal influenza A virus (IAV) infection, induction of lethal invasive GAS infections is often the result. In the present study, we established several isogenic mutants from a GAS isolate and evaluated several virulence factors as candidates responsible for the induction of invasive GAS infections. Disruption of the synthesis of the capsule, Mga, streptolysin O, streptolysin S, or streptococcal pyrogenic exotoxin B of GAS significantly reduced mortality among mice superinfected with IAV and a mutant. In addition, the number of GAS organisms adhering to IAV-infected alveolar epithelial cells was markedly reduced with the capsule-depleted mutant, although this was not the case with the other mutants. Wild-type GAS was found to bind directly to IAV particles, whereas the nonencapsulated mutant showed much less ability to bind. These results suggest that the capsule plays a key role in the invasion of host tissues by GAS following superinfection with IAV and GAS.
Figures

Similar articles
-
Pathogenic mechanisms of invasive group A Streptococcus infections by influenza virus-group A Streptococcus superinfection.Microbiol Immunol. 2018 Mar;62(3):141-149. doi: 10.1111/1348-0421.12577. Microbiol Immunol. 2018. PMID: 29377225 Review.
-
The Streptococcus pyogenes fibronectin/tenascin-binding protein PrtF.2 contributes to virulence in an influenza superinfection.Sci Rep. 2018 Aug 14;8(1):12126. doi: 10.1038/s41598-018-29714-x. Sci Rep. 2018. PMID: 30108238 Free PMC article.
-
Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice.J Virol. 2003 Apr;77(7):4104-12. doi: 10.1128/jvi.77.7.4104-4112.2003. J Virol. 2003. PMID: 12634369 Free PMC article.
-
Immunotherapy targeting the Streptococcus pyogenes M protein or streptolysin O to treat or prevent influenza A superinfection.PLoS One. 2020 Jun 23;15(6):e0235139. doi: 10.1371/journal.pone.0235139. eCollection 2020. PLoS One. 2020. PMID: 32574205 Free PMC article.
-
[Streptococcus pyogenes translocates across an epithelial barrier].Nihon Saikingaku Zasshi. 2017;72(3):213-218. doi: 10.3412/jsb.72.213. Nihon Saikingaku Zasshi. 2017. PMID: 28845032 Review. Japanese.
Cited by
-
Presence of a Prophage Determines Temperature-Dependent Capsule Production in Streptococcus pyogenes.Genes (Basel). 2016 Sep 24;7(10):74. doi: 10.3390/genes7100074. Genes (Basel). 2016. PMID: 27669311 Free PMC article.
-
Capsular sialic acid of Streptococcus suis serotype 2 binds to swine influenza virus and enhances bacterial interactions with virus-infected tracheal epithelial cells.Infect Immun. 2013 Dec;81(12):4498-508. doi: 10.1128/IAI.00818-13. Epub 2013 Sep 30. Infect Immun. 2013. PMID: 24082069 Free PMC article.
-
The Association between Invasive Group A Streptococcal Diseases and Viral Respiratory Tract Infections.Front Microbiol. 2016 Mar 21;7:342. doi: 10.3389/fmicb.2016.00342. eCollection 2016. Front Microbiol. 2016. PMID: 27047460 Free PMC article. Review.
-
Contributions of Influenza Virus Hemagglutinin and Host Immune Responses Toward the Severity of Influenza Virus: Streptococcus pyogenes Superinfections.Viral Immunol. 2018 Jul/Aug;31(6):457-469. doi: 10.1089/vim.2017.0193. Epub 2018 Jun 5. Viral Immunol. 2018. PMID: 29870311 Free PMC article.
-
GP96 Drives Exacerbation of Secondary Bacterial Pneumonia following Influenza A Virus Infection.mBio. 2021 Jun 29;12(3):e0326920. doi: 10.1128/mBio.03269-20. Epub 2021 Jun 1. mBio. 2021. PMID: 34061598 Free PMC article.
References
-
- Alouf, J. 1980. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin). Pharmacol. Ther. 11:661-717. - PubMed
-
- Baxter, F., and J. McChesney. 2000. Severe group A streptococcal infection and streptococcal toxic shock syndrome. Can. J. Anaesth. 47:1129-1140. - PubMed
-
- Brammer, T. L., H. S. Izurieta, K. Fukuda, L. M. Schmeltz, H. L. Regnery, H. E. Hall, and N. J. Cox. 2000. Surveillance for influenza—United States, 1994-95, 1995-96, and 1996-97 seasons. Morb. Mortal. Wkly. Rep. CDC Surveill. Summ. 49:13-28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

