Correct promoter control is needed for trafficking of the ring-infected erythrocyte surface antigen to the host cytosol in transfected malaria parasites
- PMID: 15385514
- PMCID: PMC517558
- DOI: 10.1128/IAI.72.10.6095-6105.2004
Correct promoter control is needed for trafficking of the ring-infected erythrocyte surface antigen to the host cytosol in transfected malaria parasites
Abstract
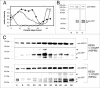
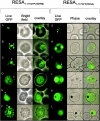
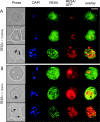
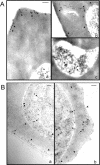
Following invasion of human erythrocytes, the malaria parasite, Plasmodium falciparum, exports proteins beyond the confines of its own plasma membrane to modify the properties of the host red cell membrane. These modifications are critical to the pathogenesis of malaria. Analysis of the P. falciparum genome sequence has identified a large number of molecules with putative atypical signal sequences. The signals remain poorly characterized; however, a number of molecules with these motifs localize to the host erythrocyte. To examine the role of these atypical signal sequences in the export of parasite proteins, we have generated transfected parasites expressing a chimeric protein comprising the N-terminal region of the P. falciparum ring-infected erythrocyte surface antigen (RESA) appended to green fluorescent protein (GFP). This N-terminal region contains a hydrophobic stretch of amino acids that is presumed to act as a noncanonical secretory signal sequence. Modulation of the timing of transgene expression demonstrates that trafficking of malaria proteins into the host erythrocyte is dependent on both the presence of an appropriate transport signal and the timing of expression. Transgene expression under the control of a trophozoite-specific promoter mistargets the chimeric molecule to the parasitophorous vacuole surrounding the parasite. However, expression of RESA-GFP in schizont stages, under the control of the RESA promoter, enables correct trafficking of a population of the chimeric protein to the host erythrocyte.
Figures


Similar articles
-
The signal sequence of exported protein-1 directs the green fluorescent protein to the parasitophorous vacuole of transfected malaria parasites.J Biol Chem. 2003 Feb 21;278(8):6532-42. doi: 10.1074/jbc.M207039200. Epub 2002 Nov 26. J Biol Chem. 2003. PMID: 12456681
-
Characterisation of a delta-COP homologue in the malaria parasite, Plasmodium falciparum.Mol Biochem Parasitol. 2002 Aug 7;123(1):11-21. doi: 10.1016/s0166-6851(02)00117-2. Mol Biochem Parasitol. 2002. PMID: 12165385
-
Trafficking determinants for PfEMP3 export and assembly under the Plasmodium falciparum-infected red blood cell membrane.Mol Microbiol. 2005 Nov;58(4):1039-53. doi: 10.1111/j.1365-2958.2005.04895.x. Mol Microbiol. 2005. PMID: 16262789
-
Vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes.Int J Parasitol. 2001 Oct;31(12):1381-91. doi: 10.1016/s0020-7519(01)00256-9. Int J Parasitol. 2001. PMID: 11566305 Review.
-
Deciphering the export pathway of malaria surface proteins.Trends Parasitol. 2006 Sep;22(9):401-4. doi: 10.1016/j.pt.2006.07.002. Epub 2006 Jul 14. Trends Parasitol. 2006. PMID: 16843728 Review.
Cited by
-
Temporal expression and localization patterns of variant surface antigens in clinical Plasmodium falciparum isolates during erythrocyte schizogony.PLoS One. 2012;7(11):e49540. doi: 10.1371/journal.pone.0049540. Epub 2012 Nov 15. PLoS One. 2012. PMID: 23166704 Free PMC article.
-
Overexpression in Plasmodium falciparum of an intrinsically disordered protein segment of PfUT impairs the parasite's proteostasis and reduces its growth rate.Front Cell Infect Microbiol. 2025 May 13;15:1565814. doi: 10.3389/fcimb.2025.1565814. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40433665 Free PMC article.
-
The C-terminal portion of the cleaved HT motif is necessary and sufficient to mediate export of proteins from the malaria parasite into its host cell.Mol Microbiol. 2013 Feb;87(4):835-50. doi: 10.1111/mmi.12133. Epub 2013 Jan 21. Mol Microbiol. 2013. PMID: 23279267 Free PMC article.
-
A genome-wide chromatin-associated nuclear peroxiredoxin from the malaria parasite Plasmodium falciparum.J Biol Chem. 2011 Apr 1;286(13):11746-55. doi: 10.1074/jbc.M110.198499. Epub 2011 Jan 31. J Biol Chem. 2011. PMID: 21282103 Free PMC article.
-
The role of KAHRP domains in knob formation and cytoadherence of P falciparum-infected human erythrocytes.Blood. 2006 Jul 1;108(1):370-8. doi: 10.1182/blood-2005-11-4624. Epub 2006 Feb 28. Blood. 2006. PMID: 16507777 Free PMC article.
References
-
- Adisa, A., M. Rug, M. Foley, and L. Tilley. 2002. Characterisation of a delta-COP homologue in the malaria parasite, Plasmodium falciparum. Mol. Biochem. Parasitol. 123:11-21. - PubMed
-
- Adisa, A., M. Rug, N. Klonis, M. Foley, A. F. Cowman, and L. Tilley. 2003. The signal sequence of exported protein-1 directs the green fluorescent protein to the parasitophorous vacuole of transfected malaria parasites. J. Biol. Chem. 278:6532-6542. - PubMed
-
- Aikawa, M., M. Torii, A. Sjolander, K. Berzins, P. Perlmann, and L. H. Miller. 1990. Pf155/RESA antigen is localized in dense granules of Plasmodium falciparum merozoites. Exp. Parasitol. 71:326-329. - PubMed
-
- Albano, F. R., M. Foley, and L. Tilley. 1999. Export of parasite proteins to the erythrocyte cytoplasm: secretory machinery and traffic signals. Novartis Found. Symp. 226:157-172. - PubMed
-
- Anders, R. F., N. Barzaga, P. T. Shi, D. B. Scanlon, L. E. Brown, L. M. Thomas, G. V. Brown, H. D. Stahl, R. L. Coppel, and D. J. Kemp. 1987. Repetitive sequences in malaria antigens, p. 333-342. In N. Agabian, H. Goodman, and N. Noguiera, Molecular strategies of parasitic invasion. UCLA Symposium on Molecular and Cellular Biology, vol. 42. Alan R. Liss, Inc., New York, N.Y.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

