Long-lasting increase of alcohol relapse by the cannabinoid receptor agonist WIN 55,212-2 during alcohol deprivation
- PMID: 15385608
- PMCID: PMC6729684
- DOI: 10.1523/JNEUROSCI.2179-04.2004
Long-lasting increase of alcohol relapse by the cannabinoid receptor agonist WIN 55,212-2 during alcohol deprivation
Abstract
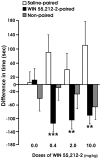
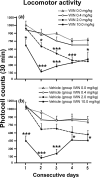
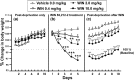
Alcoholism is characterized by successive relapses. Recent data have shown a cross-talk between the cannabinoid system and ethanol. In this study, male Wistar rats with a limited (30 min sessions), intermittent, and extended background of alcohol operant self-administration were used. The relapse to alcohol after 1 week of alcohol deprivation was evaluated. Two weeks later, the animals were treated with the cannabinoid agonist WIN 55,212-2 (R-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate) (0, 0.4, 2.0, and 10.0 mg/kg, s.c.) during a similar alcohol deprivation period, and alcohol relapse during 2 weeks was assessed. A conditioned place preference (CPP) paradigm was used to study the rewarding properties of the cannabinoid agonist. Locomotor activity was also recorded. All doses of WIN 55,212-2 produced aversion in the CPP paradigm. The doses of 2.0 and 10.0 mg/kg resulted in an important suppression of spontaneous locomotor activity and a progressive weight loss during the next 2 weeks. The single alcohol deprivation was followed by a transient increase in their responding for alcohol from a range of 20-24 lever presses at baseline to a range of 38-48 responses in the first and second days (alcohol deprivation effect). However, the administration of WIN 55,212-2 during ethanol deprivation produced similar increased responses for alcohol but in a long-term way (at least over 2 weeks). These findings suggest that noncontingent chronic exposure to cannabinoids during alcohol deprivation can potentiate the relapse into alcohol use, indicating that functional changes in the cannabinoid brain receptor may play a key role in ethanol relapse.
Figures


Similar articles
-
Cannabinoid-induced increase in relapse-like drinking is prevented by the blockade of the glycine-binding site of N-methyl-D-aspartate receptors.Neuroscience. 2009 Jan 23;158(2):465-73. doi: 10.1016/j.neuroscience.2008.10.002. Epub 2008 Oct 10. Neuroscience. 2009. PMID: 18977415
-
Effects of the cannabinoid receptor agonist WIN 55,212-2 on operant behavior and locomotor activity in rats.Pharmacol Biochem Behav. 2005 Jan;80(1):145-50. doi: 10.1016/j.pbb.2004.10.023. Epub 2004 Dec 30. Pharmacol Biochem Behav. 2005. PMID: 15652390
-
Converging action of alcohol consumption and cannabinoid receptor activation on adult hippocampal neurogenesis.Int J Neuropsychopharmacol. 2010 Mar;13(2):191-205. doi: 10.1017/S1461145709991118. Epub 2010 Jan 5. Int J Neuropsychopharmacol. 2010. PMID: 20047713
-
Modeling relapse in animals.Curr Top Behav Neurosci. 2013;13:403-32. doi: 10.1007/7854_2012_202. Curr Top Behav Neurosci. 2013. PMID: 22389178 Free PMC article. Review.
-
Animal models for studying therapeutic targets and treatments for alcohol use disorder.Int Rev Neurobiol. 2024;178:355-381. doi: 10.1016/bs.irn.2024.08.004. Epub 2024 Oct 24. Int Rev Neurobiol. 2024. PMID: 39523060 Review.
Cited by
-
CB1 receptor neutral antagonist treatment epigenetically increases neuropeptide Y expression and decreases alcohol drinking.Neuropharmacology. 2021 Sep 1;195:108623. doi: 10.1016/j.neuropharm.2021.108623. Epub 2021 May 26. Neuropharmacology. 2021. PMID: 34048869 Free PMC article.
-
Combining rimonabant and fentanyl in a single entity: preparation and pharmacological results.Drug Des Devel Ther. 2014 Feb 20;8:263-77. doi: 10.2147/DDDT.S55045. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 24591816 Free PMC article.
-
Cannabinoid modulation of drug reward and the implications of marijuana legalization.Brain Res. 2015 Dec 2;1628(Pt A):233-43. doi: 10.1016/j.brainres.2014.11.034. Epub 2014 Nov 25. Brain Res. 2015. PMID: 25463025 Free PMC article. Review.
-
Drug addiction.Curr Top Behav Neurosci. 2009;1:309-46. doi: 10.1007/978-3-540-88955-7_13. Curr Top Behav Neurosci. 2009. PMID: 21104390 Free PMC article. Review.
-
Distinct functions of endogenous cannabinoid system in alcohol abuse disorders.Br J Pharmacol. 2019 Sep;176(17):3085-3109. doi: 10.1111/bph.14780. Epub 2019 Jul 29. Br J Pharmacol. 2019. PMID: 31265740 Free PMC article. Review.
References
-
- Aceto MD, Scates SM, Martin BB (2001) Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55212-2. Eur J Pharmacol 416: 75-81. - PubMed
-
- Anggadiredja K, Yamaguchi T, Tanaka H, Shoyama Y, Watanabe S, Yamamoto T (2003) Prostaglandin E2 attenuates SR141716A-precipitated withdrawal in tetrahydrocannabinol-dependent mice. Brain Res 966: 47-53. - PubMed
-
- Basavarajappa BS, Hungund BL (1999) Down-regulation of cannabinoid receptor agonist-stimulated [35S]GTP gamma S binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res 815: 89-97. - PubMed
-
- Basavarajappa BS, Hungund BL (2002) Neuromodulatory role of the endocannabinoid signaling system in alcoholism: an overview. Prostaglandins Leukot Essent Fatty Acids 66: 287-299. - PubMed
-
- Braida D, Sala M (2000) Cannabinoid-induced working memory impairment is reversed by a second generation cholinesterase inhibitor in rats. NeuroReport 11: 2025-2029. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
