Oxidative stress activates FUS1 and RLM1 transcription in the yeast Saccharomyces cerevisiae in an oxidant-dependent Manner
- PMID: 15385622
- PMCID: PMC532035
- DOI: 10.1091/mbc.e04-02-0142
Oxidative stress activates FUS1 and RLM1 transcription in the yeast Saccharomyces cerevisiae in an oxidant-dependent Manner
Abstract
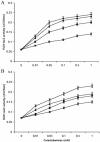
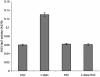
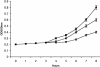
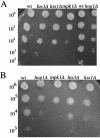
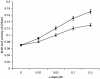
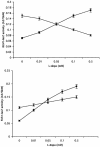
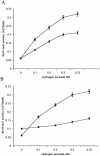
Mating in haploid Saccharomyces cerevisiae occurs after activation of the pheromone response pathway. Biochemical components of this pathway are involved in other yeast signal transduction networks. To understand more about the coordination between signaling pathways, we used a "chemical genetic" approach, searching for compounds that would activate the pheromone-responsive gene FUS1 and RLM1, a reporter for the cell integrity pathway. We found that catecholamines (l-3,4-hydroxyphenylalanine [l-dopa], dopamine, adrenaline, and noradrenaline) elevate FUS1 and RLM1 transcription. N-Acetyl-cysteine, a powerful antioxidant in yeast, completely reversed this effect, suggesting that FUS1 and RLM1 activation in response to catecholamines is a result of oxidative stress. The oxidant hydrogen peroxide also was found to activate transcription of an RLM1 reporter. Further genetic analysis combined with immunoblotting revealed that Kss1, one of the mating mitogen-activated protein kinases (MAPKs), and Mpk1, an MAPK of the cell integrity pathway, participated in l-dopa-induced stimulation of FUS1 and RLM1 transcription. We also report that Mpk1 and Hog1, the high osmolarity MAPK, were phosphorylated upon induction by hydrogen peroxide. Together, our results demonstrate that cells respond to oxidative stress via different signal transduction machinery dependent upon the nature of the oxidant.
Figures




References
-
- Basma, A.N., Nicklas, W.J., and Geller, H.M.,. (1995). L-dopa cytotoxicity to PC12 cells in culture is via its autooxidation. J. Neurochem. 64, 825-832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

