Spindle pole body duplication in fission yeast occurs at the G1/S boundary but maturation is blocked until exit from S by an event downstream of cdc10+
- PMID: 15385623
- PMCID: PMC532005
- DOI: 10.1091/mbc.e04-03-0255
Spindle pole body duplication in fission yeast occurs at the G1/S boundary but maturation is blocked until exit from S by an event downstream of cdc10+
Abstract
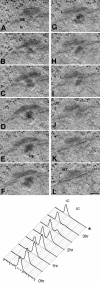
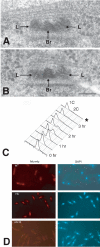
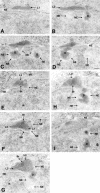
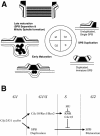
The regulation and timing of spindle pole body (SPB) duplication and maturation in fission yeast was examined by transmission electron microscopy. When cells are arrested at G1 by nitrogen starvation, the SPB is unduplicated. On release from G1, the SPBs were duplicated after 1-2 h. In cells arrested at S by hydroxyurea, SPBs are duplicated but not mature. In G1 arrest/release experiments with cdc2.33 cells at the restrictive temperature, SPBs remained single, whereas in cells at the permissive temperature, SPBs were duplicated. In cdc10 mutant cells, the SPBs seem not only to be duplicated but also to undergo partial maturation, including invagination of the nuclear envelope underneath the SPB. There may be an S-phase-specific inhibitor of SPB maturation whose expression is under control of cdc10(+). This model was examined by induction of overreplication of the genome by overexpression of rum1p or cdc18p. In cdc18p-overexpressing cells, the SPBs are duplicated but not mature, suggesting that cdc18p is one component of this feedback mechanism. In contrast, cells overexpressing rum1p have large, deformed SPBs accompanied by other features of maturation and duplication. We propose a feedback mechanism for maturation of the SPB that is coupled with exit from S to trigger morphological changes.
Figures





Similar articles
-
Fission yeast cdc31p is a component of the half-bridge and controls SPB duplication.Mol Biol Cell. 2003 Jul;14(7):2793-808. doi: 10.1091/mbc.e02-10-0661. Epub 2003 Apr 4. Mol Biol Cell. 2003. PMID: 12857865 Free PMC article.
-
Spindle pole body components are reorganized during fission yeast meiosis.Mol Biol Cell. 2012 May;23(10):1799-811. doi: 10.1091/mbc.E11-11-0951. Epub 2012 Mar 21. Mol Biol Cell. 2012. PMID: 22438582 Free PMC article.
-
Duplication of the Yeast Spindle Pole Body Once per Cell Cycle.Mol Cell Biol. 2016 Apr 15;36(9):1324-31. doi: 10.1128/MCB.00048-16. Print 2016 May. Mol Cell Biol. 2016. PMID: 26951196 Free PMC article. Review.
-
Plo1 kinase recruitment to the spindle pole body and its role in cell division in Schizosaccharomyces pombe.Mol Biol Cell. 1999 Aug;10(8):2771-85. doi: 10.1091/mbc.10.8.2771. Mol Biol Cell. 1999. PMID: 10436027 Free PMC article.
-
Big Lessons from Little Yeast: Budding and Fission Yeast Centrosome Structure, Duplication, and Function.Annu Rev Genet. 2017 Nov 27;51:361-383. doi: 10.1146/annurev-genet-120116-024733. Epub 2017 Sep 15. Annu Rev Genet. 2017. PMID: 28934593 Review.
Cited by
-
Transient structure associated with the spindle pole body directs meiotic microtubule reorganization in S. pombe.Curr Biol. 2012 Apr 10;22(7):562-74. doi: 10.1016/j.cub.2012.02.042. Epub 2012 Mar 15. Curr Biol. 2012. PMID: 22425159 Free PMC article.
-
Reorganization of the growth pattern of Schizosaccharomyces pombe in invasive filament formation.Eukaryot Cell. 2010 Nov;9(11):1788-97. doi: 10.1128/EC.00084-10. Epub 2010 Sep 24. Eukaryot Cell. 2010. PMID: 20870879 Free PMC article.
-
The S. pombe mitotic regulator Cut12 promotes spindle pole body activation and integration into the nuclear envelope.J Cell Biol. 2009 Jun 1;185(5):875-88. doi: 10.1083/jcb.200812108. J Cell Biol. 2009. PMID: 19487457 Free PMC article.
-
Mitotic chromosome biorientation in fission yeast is enhanced by dynein and a minus-end-directed, kinesin-like protein.Mol Biol Cell. 2007 Jun;18(6):2216-25. doi: 10.1091/mbc.e06-11-0987. Epub 2007 Apr 4. Mol Biol Cell. 2007. PMID: 17409356 Free PMC article.
-
Anatomy of the fungal microtubule organizing center, the spindle pole body.Curr Opin Struct Biol. 2021 Feb;66:22-31. doi: 10.1016/j.sbi.2020.09.008. Epub 2020 Oct 25. Curr Opin Struct Biol. 2021. PMID: 33113389 Free PMC article. Review.
References
-
- Braunfeld, M.B., Koster, A.J., Sedat, J.W., and Agard, D.A. (1994). Cryo automated electron tomography: towards high-resolution reconstructions of plastic-embedded structures. J. Microsc. 174, 75–84. - PubMed
-
- Chen, H., Hughes, D.D., Chan, T.A., Sedat, J.W., and Agard, D.A. (1996). IVE (Image Visualization Environment): a software platform for all three-dimensional microscopy applications. J. Struct. Biol. 116, 56–60. - PubMed
-
- Fisher, D., and Nurse, P. (1995). Cyclins of the fission yeast Schizosaccharomyces pombe. Semin. Cell Biol. 6, 73–78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

