Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts
- PMID: 15385625
- PMCID: PMC532013
- DOI: 10.1091/mbc.e04-05-0385
Characterization of the TPX2 domains involved in microtubule nucleation and spindle assembly in Xenopus egg extracts
Abstract
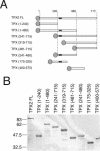
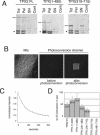
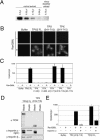
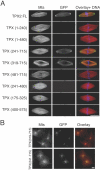
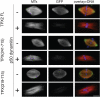
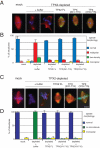
TPX2 has multiple functions during mitosis, including microtubule nucleation around the chromosomes and the targeting of Xklp2 and Aurora A to the spindle. We have performed a detailed domain functional analysis of TPX2 and found that a large N-terminal domain containing the Aurora A binding peptide interacts directly with and nucleates microtubules in pure tubulin solutions. However, it cannot substitute the endogenous TPX2 to support microtubule nucleation in response to Ran guanosine triphosphate (GTP) and spindle assembly in egg extracts. By contrast, a large C-terminal domain of TPX2 that does not bind directly to pure microtubules and does not bind Aurora A kinase rescues microtubule nucleation in response to RanGTP and spindle assembly in TPX2-depleted extract. These and previous results suggest that under physiological conditions, TPX2 is essential for microtubule nucleation around chromatin and functions in a network of other molecules, some of which also are regulated by RanGTP.
Figures



References
-
- Bayliss, R., Sardon, T., Vernos, I., and Conti, E. (2003). Structural basis of Aurora-A activation by TPX2 at the mitotic spindle. Mol. Cell 12, 851–862. - PubMed
-
- Bornens, M., and Moudjou, M. (1999). Studying the composition and function of centrosomes in vertebrates. Methods Cell Biol. 61, 13–34. - PubMed
-
- Dasso, M. (2001). Running on Ran: nuclear transport and the mitotic spindle. Cell 104, 321–324. - PubMed
-
- Dasso, M. (2002). The Ran GTPase: theme and variations. Curr. Biol. 12, R502–R508. - PubMed
-
- Desai, A., Murray, A., Mitchison, T.J., and Walczak, C.E. (1999). The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61, 385–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

