mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulum-golgi units involved in gurken transport in Drosophila oocytes
- PMID: 15385627
- PMCID: PMC532012
- DOI: 10.1091/mbc.e04-05-0398
mRNA localization and ER-based protein sorting mechanisms dictate the use of transitional endoplasmic reticulum-golgi units involved in gurken transport in Drosophila oocytes
Abstract
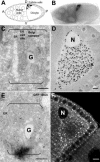
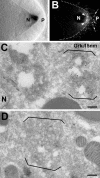
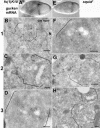
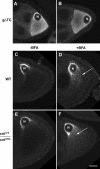
The anteroposterior and dorsoventral axes of the future embryo are specified within Drosophila oocytes by localizing gurken mRNA, which targets the secreted Gurken transforming growth factor-alpha synthesis and transport to the same site. A key question is whether gurken mRNA is targeted to a specialized exocytic pathway to achieve the polar deposition of the protein. Here, we show, by (immuno)electron microscopy that the exocytic pathway in stage 9-10 Drosophila oocytes comprises a thousand evenly distributed transitional endoplasmic reticulum (tER)-Golgi units. Using Drosophila mutants, we show that it is the localization of gurken mRNA coupled to efficient sorting of Gurken out of the ER that determines which of the numerous equivalent tER-Golgi units are used for the protein transport and processing. The choice of tER-Golgi units by mRNA localization makes them independent of each other and represents a nonconventional way, by which the oocyte implements polarized deposition of transmembrane/secreted proteins. We propose that this pretranslational mechanism could be a general way for targeted secretion in polarized cells, such as neurons.
Figures




Similar articles
-
Evidence for a transport-trap mode of Drosophila melanogaster gurken mRNA localization.PLoS One. 2010 Nov 12;5(11):e15448. doi: 10.1371/journal.pone.0015448. PLoS One. 2010. PMID: 21103393 Free PMC article.
-
Sec61beta, a subunit of the Sec61 protein translocation channel at the endoplasmic reticulum, is involved in the transport of Gurken to the plasma membrane.BMC Cell Biol. 2009 Feb 18;10:11. doi: 10.1186/1471-2121-10-11. BMC Cell Biol. 2009. PMID: 19226464 Free PMC article.
-
Translational control of gurken mRNA in Drosophila development.Cell Cycle. 2017 Jan 2;16(1):23-32. doi: 10.1080/15384101.2016.1250048. Epub 2016 Nov 14. Cell Cycle. 2017. PMID: 27841697 Free PMC article.
-
[Source of asymmetry in ontogeny: early polarization of the germline cyst and oocyte in Drosophila].Genetika. 2008 Sep;44(9):1157-71. Genetika. 2008. PMID: 18846812 Review. Russian.
-
The Role of Microtubule Motors in mRNA Localization and Patterning Within the Drosophila Oocyte.Results Probl Cell Differ. 2017;63:149-168. doi: 10.1007/978-3-319-60855-6_7. Results Probl Cell Differ. 2017. PMID: 28779317 Review.
Cited by
-
A dual role for actin and microtubule cytoskeleton in the transport of Golgi units from the nurse cells to the oocyte across ring canals.Mol Biol Cell. 2009 Jan;20(1):556-68. doi: 10.1091/mbc.e08-04-0360. Epub 2008 Nov 12. Mol Biol Cell. 2009. PMID: 19005218 Free PMC article.
-
Jagunal is required for reorganizing the endoplasmic reticulum during Drosophila oogenesis.J Cell Biol. 2007 Mar 26;176(7):941-52. doi: 10.1083/jcb.200701048. J Cell Biol. 2007. PMID: 17389229 Free PMC article.
-
The RNA-binding protein Rrm4 is essential for efficient secretion of endochitinase Cts1.Mol Cell Proteomics. 2011 Dec;10(12):M111.011213. doi: 10.1074/mcp.M111.011213. Epub 2011 Aug 1. Mol Cell Proteomics. 2011. PMID: 21808052 Free PMC article.
-
Drosophila Hephaestus/polypyrimidine tract binding protein is required for dorso-ventral patterning and regulation of signalling between the germline and soma.PLoS One. 2013 Jul 23;8(7):e69978. doi: 10.1371/journal.pone.0069978. Print 2013. PLoS One. 2013. PMID: 23894566 Free PMC article.
-
Loss of a Clueless-dGRASP complex results in ER stress and blocks Integrin exit from the perinuclear endoplasmic reticulum in Drosophila larval muscle.Biol Open. 2015 Apr 10;4(5):636-48. doi: 10.1242/bio.201511551. Biol Open. 2015. PMID: 25862246 Free PMC article.
References
-
- Altan-Bonnet, N., Sougrat, R., and Lippincott-Schwartz, J. (2004). Molecular basis for Golgi maintenance and biogenesis. Curr. Opin. Cell Biol. 16, 364–372. - PubMed
-
- Barlowe, C. (2003). Signals for COPII-dependent export from the ER: what's the ticket out? Trends Cell Biol. 13, 295–300. - PubMed
-
- Barlowe, C., Orci, L., Yeung. T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M.F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). C.O.P.II: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895–907. - PubMed
-
- Bashirullah, A., Cooperstock, R.L., and Lipshitz, H.D. (1998). RNA localization in development. Annu. Rev. Biochem. 67, 35–94. - PubMed
-
- Bobinnec, Y., Marcaillou, C., Morin, X., and Debec, A. (2003). Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster. Cell Motil. Cytoskeleton 54, 217–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

