Bipolarization and poleward flux correlate during Xenopus extract spindle assembly
- PMID: 15385629
- PMCID: PMC532038
- DOI: 10.1091/mbc.e04-05-0440
Bipolarization and poleward flux correlate during Xenopus extract spindle assembly
Abstract
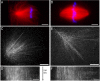
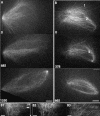
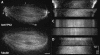
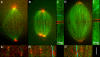
We investigated the mechanism by which meiotic spindles become bipolar and the correlation between bipolarity and poleward flux, using Xenopus egg extracts. By speckle microscopy and computational alignment, we find that monopolar sperm asters do not show evidence for flux, partially contradicting previous work. We account for the discrepancy by describing spontaneous bipolarization of sperm asters that was missed previously. During spontaneous bipolarization, onset of flux correlated with onset of bipolarity, implying that antiparallel microtubule organization may be required for flux. Using a probe for TPX2 in addition to tubulin, we describe two pathways that lead to spontaneous bipolarization, new pole assembly near chromatin, and pole splitting. By inhibiting the Ran pathway with excess importin-alpha, we establish a role for chromatin-derived, antiparallel overlap bundles in generating the sliding force for flux, and we examine these bundles by electron microscopy. Our results highlight the importance of two processes, chromatin-initiated microtubule nucleation, and sliding forces generated between antiparallel microtubules, in self-organization of spindle bipolarity and poleward flux.
Figures


References
-
- Chen, W., and Zhang, D. (2004). Kinetochore fibre dynamics outside the context of the spindle during anaphase. Nat. Cell Biol. 6, 227-231. - PubMed
-
- Desai, A., Murray, A., Mitchison, T.J., and Walczak, C.E. (1999a). The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61, 385-412. - PubMed
-
- Field, C.M., Oegema, K., Zheng, Y., Mitchison, T.J., and Walczak, C.E. (1998). Purification of cytoskeletal proteins using peptide antibodies. Methods Enzymol. 298, 525-541. - PubMed
-
- Gorlich, D., Prehn, S., Laskey, R.A., and Hartmann, E. (1994). Isolation of a protein that is essential for the first step of nuclear protein import. Cell 79, 767-778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

