Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C
- PMID: 15387887
- PMCID: PMC522750
- DOI: 10.1186/1476-5926-3-8
Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C
Abstract
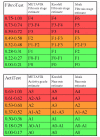
SUMMARY: BACKGROUND: Recent studies strongly suggest that due to the limitations and risks of biopsy, as well as the improvement of the diagnostic accuracy of biochemical markers, liver biopsy should no longer be considered mandatory in patients with chronic hepatitis C. In 2001, FibroTest ActiTest (FT-AT), a panel of biochemical markers, was found to have high diagnostic value for fibrosis (FT range 0.00-1.00) and necroinflammatory histological activity (AT range 0.00-1.00). The aim was to summarize the diagnostic value of these tests from the scientific literature; to respond to frequently asked questions by performing original new analyses (including the range of diagnostic values, a comparison with other markers, the impact of genotype and viral load, and the diagnostic value in intermediate levels of injury); and to develop a system of conversion between the biochemical and biopsy estimates of liver injury. RESULTS: A total of 16 publications were identified. An integrated database was constructed using 1,570 individual data, to which applied analytical recommendations. The control group consisted of 300 prospectively studied blood donors. For the diagnosis of significant fibrosis by the METAVIR scoring system, the areas under the receiver operating characteristics curves (AUROC) ranged from 0.73 to 0.87. For the diagnosis of significant histological activity, the AUROCs ranged from 0.75 to 0.86. At a cut off of 0.31, the FT negative predictive value for excluding significant fibrosis (prevalence 0.31) was 91%. At a cut off of 0.36, the ActiTest negative predictive value for excluding significant necrosis (prevalence 0.41) was 85%. In three studies there was a direct comparison in the same patients of FT versus other biochemical markers, including hyaluronic acid, the Forns index, and the APRI index. All the comparisons favored FT (P < 0.05). There were no differences between the AUROCs of FT-AT according to genotype or viral load. The AUROCs of FT-AT for consecutive stages of fibrosis and grades of necrosis were the same for both moderate and extreme stages and grades. A conversion table was constructed between the continuous FT-AT values (0.00 to 1.00) and the expected semi-quantitative fibrosis stages (F0 to F4) and necrosis grades (A0 to A3). CONCLUSIONS: Based on these results, the use of the biochemical markers of liver fibrosis (FibroTest) and necrosis (ActiTest) can be recommended as an alternative to liver biopsy for the assessment of liver injury in patients with chronic hepatitis C. In clinical practice, liver biopsy should be recommended only as a second line test, i.e., in case of high risk of error of biochemical tests.
Figures


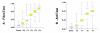
References
-
- Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
