Antimicrobial activity of euplotin C, the sesquiterpene taxonomic marker from the marine ciliate Euplotes crassus
- PMID: 15388442
- PMCID: PMC521918
- DOI: 10.1128/AAC.48.10.3828-3833.2004
Antimicrobial activity of euplotin C, the sesquiterpene taxonomic marker from the marine ciliate Euplotes crassus
Abstract
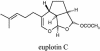
Strains of the marine ciliate protist Euplotes crassus produce exclusive terpenoids called euplotins that play an ecological role. Among these derivatives, euplotin C is the main of four secondary metabolites isolated from cultures of this protozoon and represents the sesquiterpene taxonomic marker from E. crassus. Because different terpenoid metabolites of plant origin showed a certain antimicrobial activity, we assessed the compound euplotin C, purified by high-pressure liquid chromatography and solubilized in two solubility enhancers, against the protozoa Leishmania major and Leishmani infantum, the fungus Candida albicans, and nine strains of gram-positive and gram-negative microorganisms. An activity of euplotin C against Leishmania promastigotes was demonstrated (50% lethal doses were 4.6 or 8.1 microg/ml depending on the agent used to solubilize the compound), while the effect was less evident on Candida and nearly absent on bacteria. A nonsignificant cytotoxicity (50% lethal dose, >200 microg/ml) against the J774 cell line was observed. A leishmanicidal activity was also shown by the living, euplotin-producing cells of E. crassus cultured together with promastigotes; this activity increased with time from 10 min to 6 h of incubation. This study provides an initial rationale for the evaluation of euplotin C and other similar natural products as alternative or possibly synergistic compounds for current antiprotozoon chemotherapeutics.
Figures



References
-
- Akendengue, B., F. Roblot, P. M. Loiseau, C. Bories, E. Ngou-Milama, A. Laurens, and R. Hocquemiller. 2002. Klaivanolide, an antiprotozoal lactone from Uvaria klaineana. Phytochemistry 59:885-888. - PubMed
-
- Aungst, R. A., Jr., and R. L. Funk. 2001. Synthesis of (Z)-2-acyl-2-enals via retrocycloadditions of 5-acyl-4-alkyl-4H-1,3-dioxins: application in the total synthesis of the cytotoxin (±)-euplotin A. J. Am. Chem. Soc. 123:9455-9456. - PubMed
-
- Bidwell, J., and S. Spotte. 1985. Artificial seawaters: formulas and methods. Jones and Barlett Publishers, Boston, Mass.
-
- Camacho, M. R., R. Mata, P. Castaneda, G. C. Kirby, D. C. Warhust, S. L. Croft, and J. D. Phillipson. 2000. Bioactive compounds from Celaenodendron mexicanum. Planta Med. 66:463-468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

