In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission
- PMID: 15388443
- PMCID: PMC521884
- DOI: 10.1128/AAC.48.10.3834-3844.2004
In vitro comparison of topical microbicides for prevention of human immunodeficiency virus type 1 transmission
Abstract
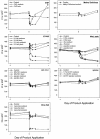
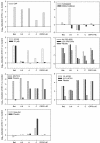
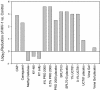
A standardized protocol was used to compare cellular toxicities and anti-human immunodeficiency virus type 1 (HIV-1) activities of candidate microbicides formulated for human use. The microbicides evaluated were cellulose acetate phthalate (CAP), Carraguard, K-Y plus nonoxynol-9 (KY-N9), PRO 2000 (0.5 and 4%), SPL7013 (5%), UC781 (0.1 and 1%), and Vena Gel, along with their accompanying placebos. Products were evaluated for toxicity on cervical and colorectal epithelial cell lines, peripheral blood mononuclear cells (PBMCs), and macrophages (MPhi) by using an ATP release assay, and they were tested for their effect on transepithelial resistance (TER) of polarized epithelial monolayers. Anti-HIV-1 activity was evaluated in assays for transfer of infectious HIV-1 from epithelial cells to activated PBMCs and for PBMC and MPhi infection. CAP, Carraguard, PRO 2000, SPL7013, and UC781 along with their placebos were 20- to 50-fold less toxic than KY-N9 and Vena Gel. None of the nontoxic product concentrations disrupted the TER. Transfer of HIV-1(Ba-L) from epithelial cells to PBMCs and PBMC and MPhi infection with laboratory-adapted HIV-1(Ba-L) and HIV-1(LAI) isolates were inhibited by all products except Carraguard, KY-N9, and Vena Gel. KY-N9, Vena Gel, and Carraguard were not effective in blocking PBMC infection with primary HIV-1(A), HIV-1(C), and HIV-1(CRF01-AE) isolates. The concordance of these toxicity results with those previously reported indicates that our protocol may be useful for predicting toxicity in vivo. Moreover, our systematic anti-HIV-1 testing provides a rational basis for making better informed decisions about which products to consider for clinical trials.
Figures

References
-
- Bader, J. P., J. B. McMahon, R. J. Schultz, V. L. Narayanan, J. B. Pierce, W. A. Harrison, O. S. Weislow, C. F. Midelfort, S. F. Stinson, and M. R. Boyd. 1991. Oxathiin carboxanilide, a potent inhibitor of human immunodeficiency virus reproduction. Proc. Natl. Acad. Sci. USA 88:6740-6744. - PMC - PubMed
-
- Balzarini, J., L. Naesens, E. Verbeken, M. Laga, L. Van Damme, M. Parniak, L. Van Mellaert, J. Anne, and E. De Clercq. 1998. Preclinical studies on thiocarboxanilide UC-781 as a virucidal agent. AIDS 12:1129-1138. - PubMed
-
- Bernstein, D. I., L. R. Stanberry, S. Sacks, N. K. Ayisi, Y. H. Gong, J. Ireland, R. J. Mumper, G. Holan, B. Matthews, T. McCarthy, and N. Bourne. 2003. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob. Agents Chemother. 47:3784-3788. - PMC - PubMed
-
- Borkow, G., H. Salomon, M. A. Wainberg, and M. A. Parniak. 2002. Attenuated infectivity of HIV type 1 from epithelial cells pretreated with a tight-binding nonnucleoside reverse transcriptase inhibitor. AIDS Res. Hum. Retrovir. 18:711-714. - PubMed
-
- Bourinbaiar, A. S., and E. C. Fruhstorfer. 1996. The efficacy of nonoxynol-9 from an in vitro point of view. AIDS 10:558-559. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

