Inhibition of human cytomegalovirus replication by benzimidazole nucleosides involves three distinct mechanisms
- PMID: 15388453
- PMCID: PMC521925
- DOI: 10.1128/AAC.48.10.3918-3927.2004
Inhibition of human cytomegalovirus replication by benzimidazole nucleosides involves three distinct mechanisms
Abstract
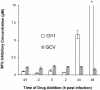
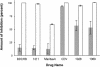
The benzimidazole nucleosides 2-bromo-5,6-dichloro-1-(beta-d-ribofuranosyl)benzimidazole (BDCRB) and 2-isopropylamino-5,6-dichloro-1-(beta-l-ribofuranosyl)benzimidazole (1263W94, or maribavir) are potent and selective inhibitors of human cytomegalovirus (HCMV) replication. These inhibitors act by two different mechanisms: BDCRB blocks the processing and maturation of viral DNA, whereas maribavir prevents viral DNA synthesis and capsid nuclear egress. In order to determine by which of these two mechanisms other benzimidazole nucleosides acted, we performed time-of-addition studies and other experiments with selected new analogs. We found that the erythrofuranosyl analog and the alpha-lyxofuranosyl analog acted late in the viral replication cycle, similar to BDCRB. In marked contrast, the alpha-5'-deoxylyxofuranosyl analog of 2,5,6-trichloro-1-(beta-d-ribofuranosyl)benzimidazole (compound UMJD1311) acted early in the replication cycle, too early to be consistent with either mechanism. Similar to other reports on early acting inhibitors of herpesviruses, compound 1311 was multiplicity of infection dependent, an observation that could not be reproduced with UV-inactivated virus. HCMV isolates resistant to BDCRB and maribavir were sensitive to compound 1311, as were viruses resistant to ganciclovir, cidofovir, and foscarnet. The preincubation of host cells with compound 1311 and removal prior to the addition of HCMV did not produce an antiviral cellular response. We conclude that this newly discovered early mode of action occurs at a stage of viral replication after entry to cells but prior to viral DNA synthesis, thereby strongly suggesting that the trisubstituted benzimidazole nucleoside series possesses three distinct biochemical modes of action for inhibition of HCMV replication.
Figures




References
-
- Alford, C. A., and W. J. Britt. 1993. Cytomegalovirus, p. 227-255. In B. Roizman, R. J. Whitley, and C. Lopez (ed.), The human herpesviruses. Raven Press, New York, N.Y.
-
- Amin, H. I., E. Ai, H. R. McDonald, and R. N. Johnson. 2000. Retinal toxic effects associated with intravitreal fomivirsen. Arch. Ophthalmol. 118:426-427. - PubMed
-
- Baldanti, F., M. R. Underwood, S. C. Stanat, K. K. Biron, S. Chou, A. Sarasini, E. Silini, and G. Gerna. 1996. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J. Virol. 70:1390-1395. - PMC - PubMed
-
- Balfour, H. H., Jr. 1979. Cytomegalovirus: the troll of transplantation. Arch. Int. Med. 139:280. - PubMed
-
- Berk, T., S. J. Gordon, H. Y. Choi, and H. S. Cooper. 1985. Cytomegalovirus infection of the colon: a possible role in the exacerbations of inflammatory bowel disease. J. Gastroenterol. 80:355-360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

