Rifalazil treats and prevents relapse of clostridium difficile-associated diarrhea in hamsters
- PMID: 15388461
- PMCID: PMC521872
- DOI: 10.1128/AAC.48.10.3975-3979.2004
Rifalazil treats and prevents relapse of clostridium difficile-associated diarrhea in hamsters
Abstract
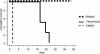
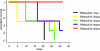
Although vancomycin and metronidazole effectively treat Clostridium difficile-associated diarrhea and colitis (CDAD), their use is associated with a high incidence of relapsing C. difficile infection. Rifalazil is a new benzoxazinorifamycin that possesses activity against Mycobacterium tuberculosis and gram-positive bacteria. Here we compared rifalazil and vancomycin for effectiveness in preventing or treating clindamycin-induced cecitis in a hamster model of CDAD. Golden Syrian hamsters were injected subcutaneously with clindamycin phosphate (10 mg/kg), followed 24 h later by C. difficile gavage. Hamsters received by gavage for 5 days vehicle, vancomycin (50 mg/kg), or rifalazil (20 mg/kg) either simultaneously with (prophylactic protocol) or 24 h after C. difficile administration (treatment protocol). While all vehicle-administered animals became moribund within 48 h of C. difficile administration, no rifalazil- or vancomycin-treated animals in either protocol showed signs of morbidity after 7 days. Ceca of rifalazil-treated animals showed absence of epithelial cell damage, significantly reduced congestion and edema, and less, but not statistically significantly less, neutrophil infiltration compared to those of vehicle-treated animals. In contrast, vancomycin-treated animals demonstrated severe epithelial cell damage and mildly reduced congestion and edema. Moreover, hamsters relapsed and tested C. difficile toxin positive (by enzyme-linked immunosorbent assay) 10 to 15 days after discontinuation of vancomycin treatment. None of the rifalazil-treated hamsters showed signs of disease or presence of toxins in their feces 30 days after discontinuation of treatment. Our results indicate that once daily rifalazil may be superior to vancomycin for curative treatment of CDAD.
Figures


Similar articles
-
Comparative efficacies of rifaximin and vancomycin for treatment of Clostridium difficile-associated diarrhea and prevention of disease recurrence in hamsters.Antimicrob Agents Chemother. 2008 Mar;52(3):1121-6. doi: 10.1128/AAC.01143-07. Epub 2008 Jan 14. Antimicrob Agents Chemother. 2008. PMID: 18195066 Free PMC article.
-
Rifamycin antibiotics for treatment of Clostridium difficile-associated diarrhea.Ann Pharmacother. 2008 Jun;42(6):827-35. doi: 10.1345/aph.1K675. Epub 2008 Apr 22. Ann Pharmacother. 2008. PMID: 18430792 Review.
-
Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin.Clin Infect Dis. 2008 Jul 1;47(1):56-62. doi: 10.1086/588293. Clin Infect Dis. 2008. PMID: 18491964
-
Oritavancin does not induce Clostridium difficile germination and toxin production in hamsters or a human gut model.J Antimicrob Chemother. 2012 Dec;67(12):2919-26. doi: 10.1093/jac/dks309. Epub 2012 Aug 16. J Antimicrob Chemother. 2012. PMID: 22899803
-
Treatment strategies for C. difficile associated diarrhea.Acta Gastroenterol Latinoam. 2007 Sep;37(3):183-91. Acta Gastroenterol Latinoam. 2007. PMID: 17955730 Review.
Cited by
-
Clinical update for the diagnosis and treatment of Clostridium difficile infection.World J Gastrointest Pharmacol Ther. 2014 Feb 6;5(1):1-26. doi: 10.4292/wjgpt.v5.i1.1. World J Gastrointest Pharmacol Ther. 2014. PMID: 24729930 Free PMC article. Review.
-
The Clinical Drug Ebselen Attenuates Inflammation and Promotes Microbiome Recovery in Mice after Antibiotic Treatment for CDI.Cell Rep Med. 2020 Apr 21;1(1):100005. doi: 10.1016/j.xcrm.2020.100005. Cell Rep Med. 2020. PMID: 32483557 Free PMC article.
-
Leaping Forward in the Treatment of Clostridium Difficile Infection: Update in 2015.GE Port J Gastroenterol. 2015 Aug 31;22(6):259-267. doi: 10.1016/j.jpge.2015.07.006. eCollection 2015 Nov-Dec. GE Port J Gastroenterol. 2015. PMID: 28868417 Free PMC article. Review.
-
Gastrointestinal localization of metronidazole by a lactobacilli-inspired tetramic acid motif improves treatment outcomes in the hamster model of Clostridium difficile infection.J Antimicrob Chemother. 2015 Nov;70(11):3061-9. doi: 10.1093/jac/dkv231. Epub 2015 Aug 18. J Antimicrob Chemother. 2015. PMID: 26286574 Free PMC article.
-
Application of recombinant antibodies for treatment of Clostridioides difficile infection: Current status and future perspective.Front Immunol. 2022 Aug 23;13:972930. doi: 10.3389/fimmu.2022.972930. eCollection 2022. Front Immunol. 2022. PMID: 36081500 Free PMC article. Review.
References
-
- Bartlett, J. G. 1981. Antimicrobial agents implicated in Clostridium difficile toxin-associated diarrhea of colitis. Johns Hopkins Med. J. 149:6-9. - PubMed
-
- Bartlett, J. G. 1984. Treatment of antibiotic-associated pseudomembranous colitis. Rev. Infect. Dis. 6:S235-S241. - PubMed
-
- Bartlett, J. G., N. Moon, T. W. Chang, N. Taylor, and A. B. Onderdonk. 1978. Role of Clostridium difficile in antibiotic-associated pseudomembranous colitis. Gastroenterology 75:778-782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

