Two year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitis
- PMID: 15388511
- PMCID: PMC1755337
- DOI: 10.1136/ard.2004.025130
Two year maintenance of efficacy and safety of infliximab in the treatment of ankylosing spondylitis
Abstract
Objective: To obtain results of the second year extension of an original 3 month randomised, placebo controlled trial (and the 1 year extension study) assessing the use of infliximab, a monoclonal antibody to tumour necrosis factor alpha, for the treatment of patients with ankylosing spondylitis (AS).
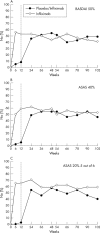
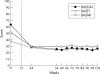
Methods: Of the 54 patients with AS who completed the first year of the study, 52 continued to receive infliximab 5 mg/kg every 6 weeks up to week 102. The primary end point was the proportion of patients achieving at least 50% improvement from baseline in the Bath AS Disease Activity Index (BASDAI) at week 102. Other assessments included patient and physician global assessments, quality of life as assessed by Short Form-36, Bath AS Functional Index, Bath AS Metrology Index, and C reactive protein (CRP).
Results: Improvement in signs and symptoms of AS seen during the first year of the study was sustained during the second year. Forty nine patients (71% of 69 enrolled patients and 49/52 (94%) patients who started year 2) completed the study up to week 102. Thirty (58%) patients achieved at least 50% improvement from baseline in the BASDAI score at week 102. Scores for other efficacy assessments were similar at weeks 54 and 102. Median CRP levels remained low at weeks 54 and 102 (3.9 and 4.3 mg/l, respectively). Side effects during the second year of the study were similar to those of the first year of treatment with infliximab.
Conclusions: Patients with AS treated for 2 years with infliximab 5 mg/kg exhibited a good and durable clinical response.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

