Efficient reversal of Alzheimer's disease fibril formation and elimination of neurotoxicity by a small molecule
- PMID: 15388848
- PMCID: PMC521943
- DOI: 10.1073/pnas.0405941101
Efficient reversal of Alzheimer's disease fibril formation and elimination of neurotoxicity by a small molecule
Abstract
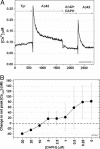
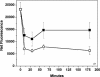
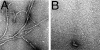
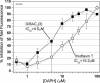
The Abeta1-42 peptide that is overproduced in Alzheimer's disease (AD) from a large precursor protein has a normal amino acid sequence but, when liberated, misfolds at neutral pH to form "protofibrils" and fibrils that are rich in beta-sheets. We find that these protofibrils or fibrils are toxic to certain neuronal cells that carry Ca-permeant alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Disrupting the structure of the Abeta1-42 fibrils and protofibrils might lead to the discovery of molecules that would be very useful in the treatment of AD. A high-throughput screen of a library of >3,000 small molecules with known "biological activity" was set up to find compounds that efficiently decrease the beta-sheet content of aggregating Abeta1-42. Lead compounds were characterized by using thioflavin T (ThT) as a beta-sheet assay. The most effective of six compounds found was 4,5-dianilinophthalimide (DAPH) under the following conditions: DAPH at low micromolar concentrations abolishes or greatly reduces previously existing fully formed Abeta1-42 fibrils, producing instead amorphous materials without fibrils but apparently containing some protofibrils and smaller forms. Coincubation of the Abeta1-42 peptide with DAPH produces either amorphous materials or empty fields. Coincubation of DAPH and Abeta1-42 greatly reduces the beta-sheet content, as measured with ThT fluorescence, and produces a novel fluorescent complex with ThT. When the Abeta1-42 peptide was coincubated with DAPH at very low micromolar concentrations, the neuronal toxicity mentioned above (Ca(2+) influx) was eliminated. Clearly, DAPH is a promising candidate for AD therapy.
Figures






References
-
- Glenner, G. G. & Wong, C. W. (1984) Biochem. Biophys. Res. Commun. 122, 1131-1135. - PubMed
-
- Sisodia, S. S., Koo, E. H., Beyreuther, K., Unterbeck, A. & Price, D. L. (1990) Science 248, 492-495. - PubMed
-
- Yankner, B. A., Duffy, L. K. & Kirschner, D. A. (1990) Science 250, 279-282. - PubMed
-
- Ferreira, A., Lu, Q., Orecchio, L. & Kosik, K. S. (1997) Mol. Cell. Neurosci. 9, 220-234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

