Comparison of TFII-I gene family members deleted in Williams-Beuren syndrome
- PMID: 15388857
- PMCID: PMC2286546
- DOI: 10.1110/ps.04747604
Comparison of TFII-I gene family members deleted in Williams-Beuren syndrome
Abstract
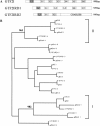
Williams-Beuren syndrome (WBS) is a neurological disorder resulting from a microdeletion, typically 1.5 megabases in size, at 7q11.23. Atypical patients implicate genes at the telomeric end of this multigene deletion as the main candidates for the pathology of WBS in particular the unequal cognitive profile associated with the condition. We recently identified a gene (GTF2IRD2) that shares homology with other members of a unique family of transcription factors (TFII-I family), which reside in the critical telomeric region. Using bioinformatics tools this study focuses on the detailed assessment of this gene family, concentrating on their characteristic structural components such as the leucine zipper (LZ) and I-repeat elements, in an attempt to identify features that could aid functional predictions. Phylogenetic analysis identified distinct I-repeat clades shared between family members. Linking functional data to one such clade has implicated them in DNA binding. The identification of PEST, synergy control motifs, and sumoylation sites common to all family members suggest a shared mechanism regulating the stability and transcriptional activity of these factors. In addition, the identification/isolation of short truncated isoforms for each TFII-I family member implies a mode of self-regulation. The exceptionally high identity shared between GTF2I and GTF2IRD2, suggests that heterodimers as well as homodimers are possible, and indicates overlapping functions between their respective short isoforms. Such cross-reactivity between GTF2I and GTF2IRD2 short isoforms might have been the evolutionary driving force for the 7q11.23 chromosomal rearrangement not present in the syntenic region in mice.
Figures



Similar articles
-
Isolation and characterisation of GTF2IRD2, a novel fusion gene and member of the TFII-I family of transcription factors, deleted in Williams-Beuren syndrome.Eur J Hum Genet. 2004 Jul;12(7):551-60. doi: 10.1038/sj.ejhg.5201174. Eur J Hum Genet. 2004. PMID: 15100712
-
GTF2IRD2 is located in the Williams-Beuren syndrome critical region 7q11.23 and encodes a protein with two TFII-I-like helix-loop-helix repeats.Proc Natl Acad Sci U S A. 2004 Jul 27;101(30):11052-7. doi: 10.1073/pnas.0404150101. Epub 2004 Jul 8. Proc Natl Acad Sci U S A. 2004. PMID: 15243160 Free PMC article.
-
A role for transcription factor GTF2IRD2 in executive function in Williams-Beuren syndrome.PLoS One. 2012;7(10):e47457. doi: 10.1371/journal.pone.0047457. Epub 2012 Oct 31. PLoS One. 2012. PMID: 23118870 Free PMC article.
-
Williams-Beuren syndrome: genes and mechanisms.Hum Mol Genet. 1999;8(10):1947-54. doi: 10.1093/hmg/8.10.1947. Hum Mol Genet. 1999. PMID: 10469848 Review.
-
Pathophysiology of TFII-I: Old Guard Wearing New Hats.Trends Mol Med. 2017 Jun;23(6):501-511. doi: 10.1016/j.molmed.2017.04.002. Epub 2017 Apr 28. Trends Mol Med. 2017. PMID: 28461154 Free PMC article. Review.
Cited by
-
Essential functions of the Williams-Beuren syndrome-associated TFII-I genes in embryonic development.Proc Natl Acad Sci U S A. 2009 Jan 6;106(1):181-6. doi: 10.1073/pnas.0811531106. Epub 2008 Dec 24. Proc Natl Acad Sci U S A. 2009. PMID: 19109438 Free PMC article.
-
Mutant GTF2I induces cell transformation and metabolic alterations in thymic epithelial cells.Cell Death Differ. 2020 Jul;27(7):2263-2279. doi: 10.1038/s41418-020-0502-7. Epub 2020 Feb 7. Cell Death Differ. 2020. PMID: 32034314 Free PMC article.
-
Neurobiology of social behavior abnormalities in autism and Williams syndrome.Nat Neurosci. 2016 Apr 26;19(6):647-655. doi: 10.1038/nn.4276. Nat Neurosci. 2016. PMID: 29323671 Free PMC article. Review.
-
Genomic and proteomic analysis of transcription factor TFII-I reveals insight into the response to cellular stress.Nucleic Acids Res. 2014 Jul;42(12):7625-41. doi: 10.1093/nar/gku467. Epub 2014 May 29. Nucleic Acids Res. 2014. PMID: 24875474 Free PMC article.
-
SUMOylation of GTF2IRD1 regulates protein partner interactions and ubiquitin-mediated degradation.PLoS One. 2012;7(11):e49283. doi: 10.1371/journal.pone.0049283. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23145142 Free PMC article.
References
-
- Altschul, S.F., Gish, W., Miller, W., Myers, E.W., and Lipman, D.J. 1990. Basic local alignment search tool. J. Mol. Biol. 215 403–410. - PubMed
-
- Baumer, A., Dutly, F., Balmer, D., Riegel, M., Tukel, T., Krajewska-Walasek, M., and Schinzel, A. 1998. High level of unequal meiotic crossovers at the origin of the 22q11.2 and 7q11.23 deletions. Hum. Mol. Genet. 7 887–894. - PubMed
-
- Bayarsaihan, D., Dunai, J., Greally, J.M., Kawasaki, K., Sumiyama, K., Enkhmandakh, B., Shimizu, N., and Ruddle, F.H. 2002. Genomic organization of the genes Gtf2ird1, Gtf2i and Ncf1 at the mouse chromosome 5 region syntenic to the human chromosome 7q11.23 Williams syndrome critical region. Genomics 79 137–143. - PubMed
-
- Bellugi, U., Lichtenberger, L., Jones, W., and Lai, Z. 2000. The neurocognitive profile of Williams syndrome: A complex pattern of strengths and weaknesses. J. Cogn. Neurosci. 12 (Suppl. 1) 7–29. - PubMed
-
- Benezra, R., Davis, R.L., Lockshun, D., Turner, D.L., and Weintraub, H. 1990. The protein Id: A negative regulator of helix-loop-helix DNA binding proteins. Cell 61 49–59. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

