Starch division and partitioning. A mechanism for granule propagation and maintenance in the picophytoplanktonic green alga Ostreococcus tauri
- PMID: 15448195
- PMCID: PMC523392
- DOI: 10.1104/pp.104.044131
Starch division and partitioning. A mechanism for granule propagation and maintenance in the picophytoplanktonic green alga Ostreococcus tauri
Abstract
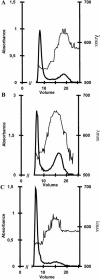
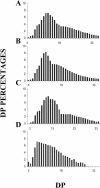
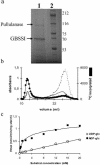
Whereas Glc is stored in small-sized hydrosoluble glycogen particles in archaea, eubacteria, fungi, and animal cells, photosynthetic eukaryotes have resorted to building starch, which is composed of several distinct polysaccharide fractions packed into a highly organized semicrystalline granule. In plants, both the initiation of polysaccharide synthesis and the nucleation mechanism leading to formation of new starch granules are currently not understood. Ostreococcus tauri, a unicellular green alga of the Prasinophyceae family, defines the tiniest eukaryote with one of the smallest genomes. We show that it accumulates a single starch granule at the chloroplast center by using the same pathway as higher plants. At the time of plastid division, we observe elongation of the starch and division into two daughter structures that are partitioned in each newly formed chloroplast. These observations suggest that in this system the information required to initiate crystalline polysaccharide growth of a new granule is contained within the preexisting polysaccharide structure and the design of the plastid division machinery.
Figures
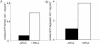


References
-
- Alonso MD, Lomako J, Lomako WM, Whelan WJ (1995) A new look at the biogenesis of glycogen. FASEB J 12: 1126–1137 - PubMed
-
- Ball S, Marianne T, Dirick L, Fresnoy M, Delrue B, Decq AA (1991) Chlamydomonas reinhardtii low-starch mutant is defective for 3-phosphoglycerate activation and orthophosphate inhibition of ADP-glucose pyrophosphorylase. Planta 185: 17–26 - PubMed
-
- Ball SG, Morell MK (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu Rev Plant Biol 54: 207–233 - PubMed
-
- Buléon A, Colonna P, Planchot V, Ball S (1998) Starch granules: structure and biosynthesis. Int J Biol Macromol 23: 85–112 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

