Bulk-solvent and hydration-shell fluctuations, similar to alpha- and beta-fluctuations in glasses, control protein motions and functions
- PMID: 15448207
- PMCID: PMC521939
- DOI: 10.1073/pnas.0405573101
Bulk-solvent and hydration-shell fluctuations, similar to alpha- and beta-fluctuations in glasses, control protein motions and functions
Abstract
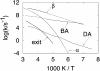
The concept that proteins exist in numerous different conformations or conformational substates, described by an energy landscape, is now accepted, but the dynamics is incompletely explored. We have previously shown that large-scale protein motions, such as the exit of a ligand from the protein interior, follow the dielectric fluctuations in the bulk solvent. Here, we demonstrate, by using mean-square displacements (msd) from Mossbauer and neutron-scattering experiments, that fluctuations in the hydration shell control fast fluctuations in the protein. We call the first type solvent-slaved or alpha-fluctuations and the second type hydration-shell-coupled or beta-fluctuations. Solvent-slaved motions are similar to the alpha-fluctuations in glasses. Their temperature dependence can be approximated by a Vogel-Tammann-Fulcher relation and they are absent in a solid environment. Hydration-shell-coupled fluctuations are similar to the beta-relaxation in glasses. They can be approximated by a Ferry or an Arrhenius relation, are much reduced or absent in dehydrated proteins, and occur in hydrated proteins even if embedded in a solid. They can be responsible for internal processes such as the migration of ligands within myoglobin. The existence of two functionally important fluctuations in proteins, one slaved to bulk motions and the other coupled to hydration-shell fluctuations, implies that the environment can control protein functions through different avenues and that no real protein transition occurs at approximately 200 K. The large number of conformational substates is essential; proteins cannot function without this reservoir of entropy, which resides mainly in the hydration shell.
Figures



References
-
- Austin, R. H., Beeson, K. W. Eisenstein, L., Frauenfelder, H. & Gunsalus, I. C. (1975) Biochemistry 14, 5355-5373. - PubMed
-
- Case, D. A. & Karplus, M. (1979) J. Mol. Biol. 132, 343-368. - PubMed
-
- Frauenfelder, H., Petsko, G. A. & Tsernoglou, D. (1979) Nature 280, 558-563. - PubMed
-
- Frauenfelder, H., Sligar, S. G. & Wolynes P. G. (1991) Science 254, 1598-1603. - PubMed
-
- Onuchic, J. N., Luthey-Schulten, Z. & Wolynes, P. G. (1997) Annu. Rev. Phys. Chem. 48, 545-600. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

