Molecular profiling reveals synaptic release machinery in Merkel cells
- PMID: 15448211
- PMCID: PMC521975
- DOI: 10.1073/pnas.0406308101
Molecular profiling reveals synaptic release machinery in Merkel cells
Abstract
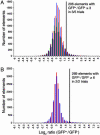
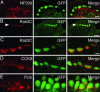
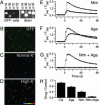
Merkel cell-neurite complexes are somatosensory receptors that initiate the perception of gentle touch. The role of epidermal Merkel cells within these complexes is disputed. To ask whether Merkel cells are genetically programmed to be excitable cells that may participate in touch reception, we purified Merkel cells from touch domes and used DNA microarrays to compare gene expression in Merkel cells and other epidermal cells. We identified 362 Merkel-cell-enriched transcripts, including neuronal transcription factors, presynaptic molecules, and ion-channel subunits. Antibody staining of skin sections showed that Merkel cells are immunoreactive for presynaptic proteins, including piccolo, Rab3C, vesicular glutamate transporter 2, and cholecystokinin 26-33. These data indicate that Merkel cells are poised to release glutamate and neuropeptides. Finally, by using Ca(2+) imaging, we discovered that Merkel cells have L- and P/Q-type voltage-gated Ca(2+) channels, which have been shown to trigger vesicle release at synapses. These results demonstrate that Merkel cells are excitable cells and suggest that they release neurotransmitters to shape touch sensitivity.
Figures


Similar articles
-
Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor.Ann N Y Acad Sci. 2013 Mar;1279(1):13-21. doi: 10.1111/nyas.12057. Ann N Y Acad Sci. 2013. PMID: 23530998 Free PMC article. Review.
-
Mechanotransduction in epidermal Merkel cells.Pflugers Arch. 2015 Jan;467(1):101-8. doi: 10.1007/s00424-014-1569-0. Epub 2014 Jul 23. Pflugers Arch. 2015. PMID: 25053537 Free PMC article. Review.
-
Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors.Nature. 2014 May 29;509(7502):617-21. doi: 10.1038/nature13250. Epub 2014 Apr 6. Nature. 2014. PMID: 24717432 Free PMC article.
-
Merkel Cells Activate Sensory Neural Pathways through Adrenergic Synapses.Neuron. 2018 Dec 19;100(6):1401-1413.e6. doi: 10.1016/j.neuron.2018.10.034. Epub 2018 Nov 8. Neuron. 2018. PMID: 30415995 Free PMC article.
-
Voltage-activated ion channels and Ca(2+)-induced Ca (2+) release shape Ca (2+) signaling in Merkel cells.Pflugers Arch. 2008 Oct;457(1):197-209. doi: 10.1007/s00424-008-0496-3. Epub 2008 Apr 16. Pflugers Arch. 2008. PMID: 18415122 Free PMC article.
Cited by
-
Evolutionary Specialization of Tactile Perception in Vertebrates.Physiology (Bethesda). 2016 May;31(3):193-200. doi: 10.1152/physiol.00036.2015. Physiology (Bethesda). 2016. PMID: 27053733 Free PMC article. Review.
-
Polycomb subunits Ezh1 and Ezh2 regulate the Merkel cell differentiation program in skin stem cells.EMBO J. 2013 Jul 17;32(14):1990-2000. doi: 10.1038/emboj.2013.110. Epub 2013 May 14. EMBO J. 2013. PMID: 23673358 Free PMC article.
-
A gut-brain neural circuit for nutrient sensory transduction.Science. 2018 Sep 21;361(6408):eaat5236. doi: 10.1126/science.aat5236. Science. 2018. PMID: 30237325 Free PMC article.
-
Dissection of Merkel cell formation in hairy and glabrous skin reveals a common requirement for FGFR2-mediated signalling.Exp Dermatol. 2019 Apr;28(4):374-382. doi: 10.1111/exd.13901. Epub 2019 Mar 13. Exp Dermatol. 2019. PMID: 30758073 Free PMC article.
-
Feeling the pressure in mammalian somatosensation.Curr Opin Neurobiol. 2005 Aug;15(4):382-8. doi: 10.1016/j.conb.2005.06.005. Curr Opin Neurobiol. 2005. PMID: 16023849 Free PMC article. Review.
References
-
- Johnson, K. O. (2001) Curr. Opin. Neurobiol. 11, 455-461. - PubMed
-
- Lumpkin, E. A., Collisson, T., Parab, P., Omer-Abdalla, A., Haeberle, H., Chen, P., Doetzlhofer, A., White, P., Groves, A., Segil, N., et al. (2003) Gene Expr. Patterns 3, 389-395. - PubMed
-
- Merkel, F. (1875) Arch. Mikrosk. Anat. 11, 636-652.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

