Bimodal speech: early suppressive visual effects in human auditory cortex
- PMID: 15450102
- PMCID: PMC1885424
- DOI: 10.1111/j.1460-9568.2004.03670.x
Bimodal speech: early suppressive visual effects in human auditory cortex
Abstract
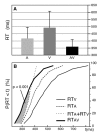
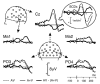
While everyone has experienced that seeing lip movements may improve speech perception, little is known about the neural mechanisms by which audiovisual speech information is combined. Event-related potentials (ERPs) were recorded while subjects performed an auditory recognition task among four different natural syllables randomly presented in the auditory (A), visual (V) or congruent bimodal (AV) condition. We found that: (i) bimodal syllables were identified more rapidly than auditory alone stimuli; (ii) this behavioural facilitation was associated with cross-modal [AV-(A+V)] ERP effects around 120-190 ms latency, expressed mainly as a decrease of unimodal N1 generator activities in the auditory cortex. This finding provides evidence for suppressive, speech-specific audiovisual integration mechanisms, which are likely to be related to the dominance of the auditory modality for speech perception. Furthermore, the latency of the effect indicates that integration operates at pre-representational stages of stimulus analysis, probably via feedback projections from visual and/or polymodal areas.
Figures




References
-
- Alcaini M, Giard MH, Thevenet M, Pernier J. Two separate frontal components in the N1 wave of the human auditory evoked response. Psychophysiology. 1994;31:611–615. - PubMed
-
- Arnold P, Hill F. Bisensory augmentation: A speechreading advantage when speech is clearly audible and intact. Br J Psychol. 2001;92:339–355. - PubMed
-
- Barth DS, Goldberg N, Brett B, Di S. The spatiotemporal organization of auditory, visual and auditory-visual evoked potentials in rat cortex. Brain Res. 1995;678:177–190. - PubMed
-
- Bense S, Stephan T, Yousry TA, Brandt T, Dieterich M. Multisensory cortical signal increases and decreases during vestibular galvanic stimulation (fMRI) J Neurophysiol. 2001;85:886–899. - PubMed
-
- Besle J, Fort A, Giard M-H. Interest and validity of the additive model in electrophysiological studies of multisensory interactions. Cognitive Processing (in press)
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

