Allosteric modulation of semicarbazide-sensitive amine oxidase activities in vitro by imidazoline receptor ligands
- PMID: 15451775
- PMCID: PMC1575421
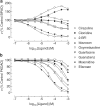
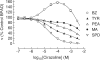
- DOI: 10.1038/sj.bjp.0705986
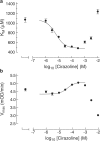
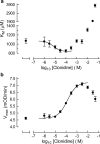
Allosteric modulation of semicarbazide-sensitive amine oxidase activities in vitro by imidazoline receptor ligands
Abstract
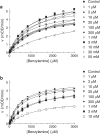
1. Evidence indicates that imidazoline I(2) binding sites (I(2)BSs) are present on monoamine oxidase (MAO) and on soluble (plasma) semicarbazide-sensitive amine oxidase enzymes. The binding site on MAO has been described as a modulatory site, although no effects on activity are thought to have been observed as a result of ligands binding to these sites. 2. We examined the effects in vitro of several imidazoline binding site ligands on activities of bovine plasma amine oxidase (BPAO) and porcine kidney diamine oxidase (PKDAO) in a spectrophotometric protocol. 3. While both enzymes were inhibited at high concentrations of all ligands, clonidine, cirazoline and oxymetazoline were seen, at lower concentrations, to increase activity of BPAO versus benzylamine, but not of PKDAO versus putrescine. This effect was substrate dependent, with mixed or biphasic inhibition of spermidine, methylamine, p-tyramine and beta-phenylethylamine oxidation observed at cirazoline concentrations that increased benzylamine oxidation. 4. With benzylamine as substrate, clonidine decreased K(M) (EC(50) 8.82 microm, E(max) 75.1% of control) and increased V(max) (EC(50) 164.6 microm, E(max) 154.1% of control). Cirazoline decreased V(max) (EC(50) 2.15 microm, E(max) 91.4% of control), then decreased K(M) (EC(50) 5.63 microm, E(max) 42.6% of control) and increased V(max) (EC(50) 49.0 microm, E(max) 114.4% of decreased V(max) value). 5. Data for clonidine fitted a mathematical model for two-site nonessential activation plus linear intersecting noncompetitive inhibition. Data for cirazoline were consistent with involvement of a fourth site. 6. These results reveal an ability of imidazoline ligands to modulate BPAO kinetics allosterically. The derived mechanism may have functional significance with respect to modulation of MAO by I(2)BS ligands.
Figures



Similar articles
-
Inhibition of monoamine oxidase A and B activities by imidazol(ine)/guanidine drugs, nature of the interaction and distinction from I2-imidazoline receptors in rat liver.Br J Pharmacol. 1997 Jul;121(5):901-12. doi: 10.1038/sj.bjp.0701214. Br J Pharmacol. 1997. PMID: 9222546 Free PMC article.
-
The effects of chronic administration of inhibitors of flavin and quinone amine oxidases on imidazoline I(1) receptor density in rat whole brain.Ann N Y Acad Sci. 2003 Dec;1009:309-22. doi: 10.1196/annals.1304.040. Ann N Y Acad Sci. 2003. PMID: 15028605
-
Metabolism of agmatine (clonidine-displacing substance) by diamine oxidase and the possible implications for studies of imidazoline receptors.Prog Brain Res. 1995;106:187-97. doi: 10.1016/s0079-6123(08)61215-7. Prog Brain Res. 1995. PMID: 8584654
-
Selective inhibitors of membrane-bound semicarbazide-sensitive amine oxidase (SSAO) activity in mammalian tissues.Neurotoxicology. 2004 Jan;25(1-2):325-35. doi: 10.1016/S0161-813X(03)00118-9. Neurotoxicology. 2004. PMID: 14697907 Review.
-
Imidazoline binding sites on receptors and enzymes: emerging targets for novel antidepressant drugs?J Psychiatry Neurosci. 2003 Nov;28(6):409-14. J Psychiatry Neurosci. 2003. PMID: 14631453 Free PMC article. Review. No abstract available.
Cited by
-
Methylxanthines Inhibit Primary Amine Oxidase and Monoamine Oxidase Activities of Human Adipose Tissue.Medicines (Basel). 2020 Apr 2;7(4):18. doi: 10.3390/medicines7040018. Medicines (Basel). 2020. PMID: 32252407 Free PMC article.
-
Opipramol Inhibits Lipolysis in Human Adipocytes without Altering Glucose Uptake and Differently from Antipsychotic and Antidepressant Drugs with Adverse Effects on Body Weight Control.Pharmaceuticals (Basel). 2020 Mar 5;13(3):41. doi: 10.3390/ph13030041. Pharmaceuticals (Basel). 2020. PMID: 32151075 Free PMC article.
-
The effects of buffer cations on interactions between mammalian copper-containing amine oxidases and their substrates.J Neural Transm (Vienna). 2007;114(6):733-41. doi: 10.1007/s00702-007-0680-1. Epub 2007 Mar 31. J Neural Transm (Vienna). 2007. PMID: 17401532
References
-
- BINDA C., NEWTON-VINSON P., HUBALEK F., EDMONDSON D.E., MATTEVI A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2001;26:22–26. - PubMed
-
- BOUSQUET P., FELDMAN J. Drugs acting on imidazoline receptors: a review of their pharmacology, their use in blood pressure control and their potential interest in cardioprotection. Drugs. 1999;58:799–812. - PubMed
-
- BOUSQUET P., FELDMAN J., SCHWARTZ J. Central cardiovascular effects of alpha adrenergic drugs: differences between catecholamines and imidazolines. J. Pharmacol. Exp. Ther. 1984;230:232–236. - PubMed
-
- CALLINGHAM B.A., HOLT A., ELLIOTT J. Properties and functions of the semicarbazide-sensitive amine oxidases. Biochem. Soc. Trans. 1991;19:228–233. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

