Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research
- PMID: 15451835
- PMCID: PMC517858
- DOI: 10.1503/cmaj.1041086
Outcome reporting bias in randomized trials funded by the Canadian Institutes of Health Research
Abstract
Background: The reporting of outcomes within published randomized trials has previously been shown to be incomplete, biased and inconsistent with study protocols. We sought to determine whether outcome reporting bias would be present in a cohort of government-funded trials subjected to rigorous peer review.
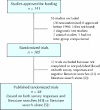
Methods: We compared protocols for randomized trials approved for funding by the Canadian Institutes of Health Research (formerly the Medical Research Council of Canada) from 1990 to 1998 with subsequent reports of the trials identified in journal publications. Characteristics of reported and unreported outcomes were recorded from the protocols and publications. Incompletely reported outcomes were defined as those with insufficient data provided in publications for inclusion in meta-analyses. An overall odds ratio measuring the association between completeness of reporting and statistical significance was calculated stratified by trial. Finally, primary outcomes specified in trial protocols were compared with those reported in publications.
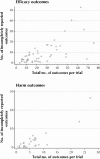
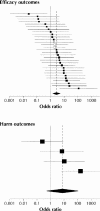
Results: We identified 48 trials with 68 publications and 1402 outcomes. The median number of participants per trial was 299, and 44% of the trials were published in general medical journals. A median of 31% (10th-90th percentile range 5%-67%) of outcomes measured to assess the efficacy of an intervention (efficacy outcomes) and 59% (0%-100%) of those measured to assess the harm of an intervention (harm outcomes) per trial were incompletely reported. Statistically significant efficacy outcomes had a higher odds than nonsignificant efficacy outcomes of being fully reported (odds ratio 2.7; 95% confidence interval 1.5-5.0). Primary outcomes differed between protocols and publications for 40% of the trials.
Interpretation: Selective reporting of outcomes frequently occurs in publications of high-quality government-funded trials.
Figures
Comment in
-
Registering CIHR-funded randomized controlled trials: a global public good.CMAJ. 2004 Sep 28;171(7):750-1. doi: 10.1503/cmaj.1041299. CMAJ. 2004. PMID: 15451838 Free PMC article. No abstract available.
-
Outcome reporting bias in government-funded RCTs.CMAJ. 2005 Mar 29;172(7):857; author reply 857. doi: 10.1503/cmaj.1041669. CMAJ. 2005. PMID: 15795393 Free PMC article. No abstract available.
References
-
- Chan AW, Haahr MT, Hróbjartsson A, Gøtzsche PC, Altman DG. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. JAMA 2004;291:2457-65. - PubMed
-
- Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases [review]. Health Technol Assess 2000;4(10):1-115. - PubMed
-
- Whitehead A. Meta-analysis of controlled clinical trials. Chichester (UK): Wiley; 2002. p. 216.
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors. Systematic reviews in healthcare. Meta-analysis in context. 2nd ed. London (UK): BMJ Books; 2001. p. 285-312.
-
- McCormack K, Scott NW, Grant AM. Outcome reporting bias and individual patient data meta-analysis: a case study in surgery [abstract]. In: Abstracts for workshops and scientific sessions. 9th International Cochrane Colloquium; Lyon; 2001. p. 34-5.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
