Altered amyloid-beta metabolism and deposition in genomic-based beta-secretase transgenic mice
- PMID: 15452128
- PMCID: PMC2659546
- DOI: 10.1074/jbc.M409680200
Altered amyloid-beta metabolism and deposition in genomic-based beta-secretase transgenic mice
Abstract
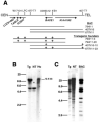
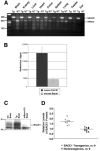
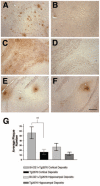
Amyloid-beta (Abeta) the primary component of the senile plaques found in Alzheimer's disease (AD) is generated by the rate-limiting cleavage of amyloid precursor protein (APP) by beta-secretase followed by gamma-secretase cleavage. Identification of the primary beta-secretase gene, BACE1, provides a unique opportunity to examine the role this unique aspartyl protease plays in altering Abeta metabolism and deposition that occurs in AD. The current experiments seek to examine how modulating beta-secretase expression and activity alters APP processing and Abeta metabolism in vivo. Genomic-based BACE1 transgenic mice were generated that overexpress human BACE1 mRNA and protein. The highest expressing BACE1 transgenic line was mated to transgenic mice containing human APP transgenes. Our biochemical and histochemical studies demonstrate that mice overexpressing both BACE1 and APP show specific alterations in APP processing and age-dependent Abeta deposition. We observed elevated levels of Abeta isoforms as well as significant increases of Abeta deposits in these double transgenic animals. In particular, the double transgenics exhibited a unique cortical deposition profile, which is consistent with a significant increase of BACE1 expression in the cortex relative to other brain regions. Elevated BACE1 expression coupled with increased deposition provides functional evidence for beta-secretase as a primary effector in regional amyloid deposition in the AD brain. Our studies demonstrate, for the first time, that modulation of BACE1 activity may play a significant role in AD pathogenesis in vivo.
Figures



References
-
- Glenner GG, Wong CW. Biochem. Biophys. Res. Commun. 1984;120:885–890. - PubMed
-
- Braak H, Braak E. Neurobiol. Aging. 1997;18:351–357. - PubMed
-
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Science. 1999;286:735–741. - PubMed
-
- Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. Nat. Neurosci. 2001;4:233–234. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

