The loss of PTEN allows TCR alphabeta lineage thymocytes to bypass IL-7 and Pre-TCR-mediated signaling
- PMID: 15452180
- PMCID: PMC2213281
- DOI: 10.1084/jem.20040495
The loss of PTEN allows TCR alphabeta lineage thymocytes to bypass IL-7 and Pre-TCR-mediated signaling
Abstract
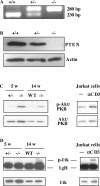
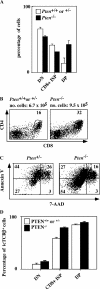
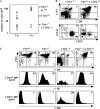
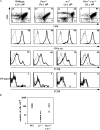
The phosphatase and tensin homologue deleted on chromosome 10 (PTEN) negatively regulates cell survival and proliferation mediated by phosphoinositol 3 kinases. We have explored the role of the phosphoinositol(3,4,5)P3-phosphatase PTEN in T cell development by analyzing mice with a T cell-specific deletion of PTEN. Pten(flox/flox)Lck-Cre mice developed thymic lymphomas, but before the onset of tumors, they showed normal thymic cellularity. To reveal a regulatory role of PTEN in proliferation of developing T cells we have crossed PTEN-deficient mice with mice deficient for interleukin (IL)-7 receptor and pre-T cell receptor (TCR) signaling. Analysis of mice deficient for Pten and CD3gamma; Pten and gammac; or Pten, gammac, and Rag2 revealed that deletion of PTEN can substitute for both IL-7 and pre-TCR signals. These double- and triple-deficient mice all develop normal levels of CD4CD8 double negative and double positive thymocytes. These data indicate that PTEN is an important regulator of proliferation of developing T cells in the thymus.
Figures


References
-
- Godfrey, D.I., and A. Zlotnik. 1993. Control points in early T-cell development. Immunol. Today. 14:547–553. - PubMed
-
- Mallick, C.A., E.C. Dudley, J.L. Viney, M.J. Owen, and A.C. Hayday. 1993. Rearrangement and diversity of T cell receptor beta chain genes in thymocytes: a critical role for the beta chain in development. Cell. 73:513–519. - PubMed
-
- von Boehmer, H., I. Aifantis, J. Feinberg, O. Lechner, C. Saint-Ruf, U. Walter, J. Buer, and O. Azogui. 1999. Pleiotropic changes controlled by the pre-T-cell receptor. Curr. Opin. Immunol. 11:135–142. - PubMed
-
- Gomez, M., V. Tybulewicz, and D.A. Cantrell. 2000. Control of pre-T cell proliferation and differentiation by the GTPase Rac-I. Nat. Immunol. 1:348–352. - PubMed
-
- Kim, K., C.K. Lee, T.J. Sayers, K. Muegge, and S.K. Durum. 1998. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. J. Immunol. 160:5735–5741. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

