Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins
- PMID: 15452181
- PMCID: PMC2213288
- DOI: 10.1084/jem.20040402
Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins
Abstract
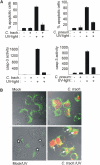
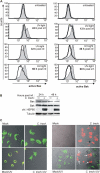
Chlamydia are obligate intracellular bacteria that replicate in a vacuole inside a host cell. Chlamydial infection has been shown to protect the host cell against apoptotic stimuli. This is likely important for the ability of Chlamydia to reproduce in human cells. Here we show that resistance to apoptosis is conveyed by the destruction of the proapoptotic BH3-only proteins Bim/Bod, Puma, and Bad during infection. Apoptotic stimuli were blocked upstream of the mitochondrial activation of Bax/Bak. During infection with both species, Chlamydia trachomatis and Chlamydia pneumoniae, Bim protein gradually disappeared without noticeable changes in Bim mRNA. The disappearance was blocked by inhibitors of the proteasome. Infected cells retained sensitivity to Bim expressed by transfection, indicating functional relevance of the Bim disappearance. Fusion to Bim targeted the green fluorescent protein for destruction during infection. Analysis of truncation mutants showed that a short region of Bim containing the BH3 domain was sufficient for destruction during chlamydial infection. Like Bim, Puma and Bad proteins disappeared during infection. These results reveal a novel way by which microbes can interfere with the host cell's apoptotic machinery, and provide a molecular explanation of the cellular resistance to apoptosis during infection with Chlamydia.
Figures




References
-
- Grayston, J.T., and S. Wang. 1975. New knowledge of chlamydiae and the diseases they cause. J. Infect. Dis. 132:87–105. - PubMed
-
- Grayston, J.T. 1992. Chlamydia pneumoniae, strain TWAR pneumonia. Annu. Rev. Med. 43:317–323. - PubMed
-
- Vaux, D.L., G. Haecker, and A. Strasser. 1994. An evolutionary perspective on apoptosis. Cell. 76:777–779. - PubMed
-
- Roulston, A., R.C. Marcellus, and P.E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577–628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

