New insights on the voltage dependence of the KCa3.1 channel block by internal TBA
- PMID: 15452196
- PMCID: PMC2233899
- DOI: 10.1085/jgp.200409145
New insights on the voltage dependence of the KCa3.1 channel block by internal TBA
Abstract
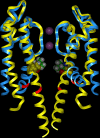
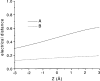
We present in this work a structural model of the open IKCa (KCa3.1) channel derived by homology modeling from the MthK channel structure, and used this model to compute the transmembrane potential profile along the channel pore. This analysis showed that the selectivity filter and the region extending from the channel inner cavity to the internal medium should respectively account for 81% and 16% of the transmembrane potential difference. We found however that the voltage dependence of the IKCa block by the quaternary ammonium ion TBA applied internally is compatible with an apparent electrical distance delta of 0.49 +/- 0.02 (n = 6) for negative potentials. To reconcile this observation with the electrostatic potential profile predicted for the channel pore, we modeled the IKCa block by TBA assuming that the voltage dependence of the block is governed by both the difference in potential between the channel cavity and the internal medium, and the potential profile along the selectivity filter region through an effect on the filter ion occupancy states. The resulting model predicts that delta should be voltage dependent, being larger at negative than positive potentials. The model also indicates that raising the internal K+ concentration should decrease the value of delta measured at negative potentials independently of the external K+ concentration, whereas raising the external K+ concentration should minimally affect delta for concentrations >50 mM. All these predictions are born out by our current experimental results. Finally, we found that the substitutions V275C and V275A increased the voltage sensitivity of the TBA block, suggesting that TBA could move further into the pore, thus leading to stronger interactions between TBA and the ions in the selectivity filter. Globally, these results support a model whereby the voltage dependence of the TBA block in IKCa is mainly governed by the voltage dependence of the ion occupancy states of the selectivity filter.
Figures








Similar articles
-
Unique inner pore properties of BK channels revealed by quaternary ammonium block.J Gen Physiol. 2004 Jul;124(1):43-57. doi: 10.1085/jgp.200409067. Epub 2004 Jun 14. J Gen Physiol. 2004. PMID: 15197222 Free PMC article.
-
Mechanism of the voltage sensitivity of IRK1 inward-rectifier K+ channel block by the polyamine spermine.J Gen Physiol. 2005 Apr;125(4):413-26. doi: 10.1085/jgp.200409242. Epub 2005 Mar 14. J Gen Physiol. 2005. PMID: 15795311 Free PMC article.
-
External TEA block of shaker K+ channels is coupled to the movement of K+ ions within the selectivity filter.J Gen Physiol. 2003 Aug;122(2):239-46. doi: 10.1085/jgp.200308848. J Gen Physiol. 2003. PMID: 12885878 Free PMC article.
-
Small conductance calcium-activated potassium channels: from structure to function.Prog Neurobiol. 2010 Jul;91(3):242-55. doi: 10.1016/j.pneurobio.2010.03.002. Epub 2010 Mar 30. Prog Neurobiol. 2010. PMID: 20359520 Review.
-
Permeation in potassium channels: implications for channel structure.Annu Rev Biophys Biophys Chem. 1987;16:227-46. doi: 10.1146/annurev.bb.16.060187.001303. Annu Rev Biophys Biophys Chem. 1987. PMID: 2439096 Review.
Cited by
-
Accessibility of four arginine residues on the S4 segment of the Bacillus halodurans sodium channel.J Membr Biol. 2007 Feb;215(2-3):169-80. doi: 10.1007/s00232-007-9016-1. Epub 2007 Jun 14. J Membr Biol. 2007. PMID: 17568977
-
Structural determinants of the closed KCa3.1 channel pore in relation to channel gating: results from a substituted cysteine accessibility analysis.J Gen Physiol. 2007 Apr;129(4):299-315. doi: 10.1085/jgp.200609726. Epub 2007 Mar 12. J Gen Physiol. 2007. PMID: 17353352 Free PMC article.
-
Aromatic-aromatic interactions between residues in KCa3.1 pore helix and S5 transmembrane segment control the channel gating process.J Gen Physiol. 2014 Feb;143(2):289-307. doi: 10.1085/jgp.201311097. J Gen Physiol. 2014. PMID: 24470490 Free PMC article.
-
Contribution of the KCa3.1 channel-calmodulin interactions to the regulation of the KCa3.1 gating process.J Gen Physiol. 2013 Jul;142(1):37-60. doi: 10.1085/jgp.201210933. J Gen Physiol. 2013. PMID: 23797421 Free PMC article.
-
Characterizing the fatty acid binding site in the cavity of potassium channel KcsA.Biochemistry. 2012 Oct 9;51(40):7996-8002. doi: 10.1021/bi3009196. Epub 2012 Sep 25. Biochemistry. 2012. PMID: 22971149 Free PMC article.
References
-
- Choi, K.L., C. Mossman, J. Aube, and G. Yellen. 1993. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron. 10:533–541. - PubMed
-
- Doyle, D.A., J.M. Cabral, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. - PubMed

