Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: implications for channel gating
- PMID: 15452199
- PMCID: PMC2233906
- DOI: 10.1085/jgp.200308973
Identification of the Ca2+ blocking site of acid-sensing ion channel (ASIC) 1: implications for channel gating
Abstract
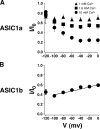
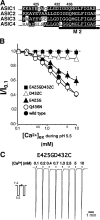
Acid-sensing ion channels ASIC1a and ASIC1b are ligand-gated ion channels that are activated by H+ in the physiological range of pH. The apparent affinity for H+ of ASIC1a and 1b is modulated by extracellular Ca2+ through a competition between Ca2+ and H+. Here we show that, in addition to modulating the apparent H+ affinity, Ca2+ blocks ASIC1a in the open state (IC50 approximately 3.9 mM at pH 5.5), whereas ASIC1b is blocked with reduced affinity (IC50 > 10 mM at pH 4.7). Moreover, we report the identification of the site that mediates this open channel block by Ca2+. ASICs have two transmembrane domains. The second transmembrane domain M2 has been shown to form the ion pore of the related epithelial Na+ channel. Conserved topology and high homology in M2 suggests that M2 forms the ion pore also of ASICs. Combined substitution of an aspartate and a glutamate residue at the beginning of M2 completely abolished block by Ca2+ of ASIC1a, showing that these two amino acids (E425 and D432) are crucial for Ca2+ block. It has previously been suggested that relief of Ca2+ block opens ASIC3 channels. However, substitutions of E425 or D432 individually or in combination did not open channels constitutively and did not abolish gating by H+ and modulation of H+ affinity by Ca2+. These results show that channel block by Ca2+ and H+ gating are not intrinsically linked.
Figures




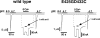
Similar articles
-
Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent.J Gen Physiol. 2006 Mar;127(3):267-76. doi: 10.1085/jgp.200509409. Epub 2006 Feb 14. J Gen Physiol. 2006. PMID: 16505147 Free PMC article.
-
The tarantula toxin psalmotoxin 1 inhibits acid-sensing ion channel (ASIC) 1a by increasing its apparent H+ affinity.J Gen Physiol. 2005 Jul;126(1):71-9. doi: 10.1085/jgp.200509303. Epub 2005 Jun 13. J Gen Physiol. 2005. PMID: 15955877 Free PMC article.
-
Gating of acid-sensitive ion channel-1: release of Ca2+ block vs. allosteric mechanism.J Gen Physiol. 2006 Feb;127(2):109-17. doi: 10.1085/jgp.200509396. Epub 2006 Jan 17. J Gen Physiol. 2006. PMID: 16418400 Free PMC article.
-
Peptides inhibitors of acid-sensing ion channels.Toxicon. 2007 Feb;49(2):271-84. doi: 10.1016/j.toxicon.2006.09.026. Epub 2006 Oct 4. Toxicon. 2007. PMID: 17113616 Review.
-
[Functional analysis of acid sensing ion channels].Nihon Shinkei Seishin Yakurigaku Zasshi. 2004 Aug;24(4):243-6. Nihon Shinkei Seishin Yakurigaku Zasshi. 2004. PMID: 15484827 Review. Japanese.
Cited by
-
Neurotoxic unc-8 mutants encode constitutively active DEG/ENaC channels that are blocked by divalent cations.J Gen Physiol. 2013 Aug;142(2):157-69. doi: 10.1085/jgp.201310974. J Gen Physiol. 2013. PMID: 23898007 Free PMC article.
-
The bile acid-sensitive ion channel is gated by Ca2+-dependent conformational changes in the transmembrane domain.bioRxiv [Preprint]. 2025 Jan 11:2025.01.10.632481. doi: 10.1101/2025.01.10.632481. bioRxiv. 2025. Update in: Nat Commun. 2025 Jul 22;16(1):6746. doi: 10.1038/s41467-025-62038-9. PMID: 39829759 Free PMC article. Updated. Preprint.
-
The bile acid-sensitive ion channel is gated by Ca2+-dependent conformational changes in the transmembrane domain.Nat Commun. 2025 Jul 22;16(1):6746. doi: 10.1038/s41467-025-62038-9. Nat Commun. 2025. PMID: 40695804 Free PMC article.
-
Molecular cloning and functional characterization of the Aplysia FMRFamide-gated Na+ channel.Pflugers Arch. 2006 Feb;451(5):646-56. doi: 10.1007/s00424-005-1498-z. Epub 2005 Aug 23. Pflugers Arch. 2006. PMID: 16133260
-
The ASIC3/P2X3 cognate receptor is a pain-relevant and ligand-gated cationic channel.Nat Commun. 2018 Apr 10;9(1):1354. doi: 10.1038/s41467-018-03728-5. Nat Commun. 2018. PMID: 29636447 Free PMC article.
References
-
- Akopian, A.N., C.C. Chen, Y. Ding, P. Cesare, and J.N. Wood. 2000. A new member of the acid-sensing ion channel family. Neuroreport. 11:2217–2222. - PubMed
-
- Alvarez de la Rosa, D., C.M. Canessa, G.K. Fyfe, and P. Zhang. 2000. Structure and regulation of amiloride-sensitive sodium channels. Annu. Rev. Physiol. 62:573–594. - PubMed
-
- Babini, E., M. Paukert, H.S. Geisler, and S. Gründer. 2002. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J. Biol. Chem. 277:41597–41603. - PubMed
-
- Bässler, E.L., T.J. Ngo-Anh, H.S. Geisler, J.P. Ruppersberg, and S. Gründer. 2001. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J. Biol. Chem. 276:33782–33787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

