Gene silencing in alveolar type II cells using cell-specific promoter in vitro and in vivo
- PMID: 15452203
- PMCID: PMC521678
- DOI: 10.1093/nar/gnh129
Gene silencing in alveolar type II cells using cell-specific promoter in vitro and in vivo
Abstract
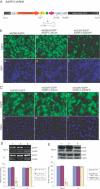
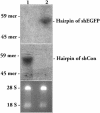
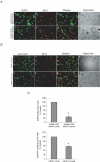
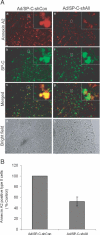
RNA interference (RNAi) is a sequence-specific post-transcriptional gene silencing process. Although it is widely used in the loss-of-function studies, none of the current RNAi technologies can achieve cell-specific gene silencing. The lack of cell specificity limits its usage in vivo. Here, we report a cell-specific RNAi system using an alveolar epithelial type II cell-specific promoter--the surfactant protein C (SP-C) promoter. We show that the SP-C-driven small hairpin RNAs specifically depress the expression of the exogenous reporter (enhanced green fluorescent protein) and endogenous genes (lamin A/C and annexin A2) in alveolar type II cells, but not other lung cells, using cell and organ culture in vitro as well as in vivo. The present study provides an efficient strategy in silencing a gene in one type of cell without interfering with other cell systems, and may have a significant impact on RNAi therapy.
Figures
Similar articles
-
Type II pneumocyte-restricted green fluorescent protein expression after lentiviral transduction of lung epithelial cells.Hum Gene Ther. 2008 Jan;19(1):39-52. doi: 10.1089/hum.2006.0180. Hum Gene Ther. 2008. PMID: 18052721
-
Surfactant protein-C chromatin-bound green fluorescence protein reporter mice reveal heterogeneity of surfactant protein C-expressing lung cells.Am J Respir Cell Mol Biol. 2013 Mar;48(3):288-98. doi: 10.1165/rcmb.2011-0403OC. Epub 2012 Nov 29. Am J Respir Cell Mol Biol. 2013. PMID: 23204392 Free PMC article.
-
In vitro and in vivo transfer and expression of human surfactant SP-A- and SP-B-associated protein cDNAs mediated by replication-deficient, recombinant adenoviral vectors.Hum Gene Ther. 1995 Mar;6(3):277-87. doi: 10.1089/hum.1995.6.3-277. Hum Gene Ther. 1995. PMID: 7779911
-
Differential expression of a V-type ATPase C subunit gene, Atp6v1c2, during culture of rat lung type II pneumocytes.J Biomed Sci. 2005 Dec;12(6):899-911. doi: 10.1007/s11373-005-9020-3. Epub 2005 Nov 9. J Biomed Sci. 2005. PMID: 16283434
-
[Regulation of alveolar type II cell proliferation and surfactant gene expression].Nihon Kyobu Shikkan Gakkai Zasshi. 1994 Dec;32 Suppl:73-8. Nihon Kyobu Shikkan Gakkai Zasshi. 1994. PMID: 7602847 Review. Japanese.
Cited by
-
An RNA polymerase II construct synthesizes short-hairpin RNA with a quantitative indicator and mediates highly efficient RNAi.Nucleic Acids Res. 2005 Apr 1;33(6):e62. doi: 10.1093/nar/gni061. Nucleic Acids Res. 2005. PMID: 15805121 Free PMC article.
-
Short hairpin RNA interference therapy for ischemic heart disease.Circulation. 2008 Sep 30;118(14 Suppl):S226-33. doi: 10.1161/CIRCULATIONAHA.107.760785. Circulation. 2008. PMID: 18824759 Free PMC article.
-
MicroRNA-127 modulates fetal lung development.Physiol Genomics. 2009 May 13;37(3):268-78. doi: 10.1152/physiolgenomics.90268.2008. Epub 2009 Mar 17. Physiol Genomics. 2009. PMID: 19439715 Free PMC article.
-
(Pro)renin receptor contributes to regulation of renal epithelial sodium channel.J Hypertens. 2016 Mar;34(3):486-94; discussion 494. doi: 10.1097/HJH.0000000000000825. J Hypertens. 2016. PMID: 26771338 Free PMC article.
-
Characterization of the cell of origin for small cell lung cancer.Cell Cycle. 2011 Aug 15;10(16):2806-15. doi: 10.4161/cc.10.16.17012. Epub 2011 Aug 15. Cell Cycle. 2011. PMID: 21822053 Free PMC article.
References
-
- Hannon G.J. (2002) RNA interference. Nature, 418, 244–251. - PubMed
-
- Elbashir S.M., Harborth,J., Lendeckel,W., Yalcin,A., Weber,K. and Tuschl,T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature, 411, 494–498. - PubMed
-
- Xia H., Mao,Q., Paulson,H.L. and Davidson,B.L. (2002) siRNA-mediated gene silencing in vitro and in vivo. Nat. Biotechnol., 20, 1006–1010. - PubMed
-
- Brummelkamp T.R., Bernards,R. and Agami,R. (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science, 296, 550–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

