Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells
- PMID: 15452205
- PMCID: PMC521813
- DOI: 10.1128/JVI.78.20.10848-10855.2004
Lentivirus-mediated RNA interference of DC-SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells
Abstract
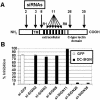
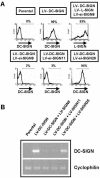
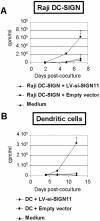
In the early events of human immunodeficiency virus type 1 (HIV-1) infection, immature dendritic cells (DCs) expressing the DC-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN) receptor capture small amounts of HIV-1 on mucosal surfaces and spread viral infection to CD4(+) T cells in lymph nodes (22, 34, 45). RNA interference has emerged as a powerful tool to gain insight into gene function. For this purpose, lentiviral vectors that express short hairpin RNA (shRNA) for the delivery of small interfering RNA (siRNA) into mammalian cells represent a powerful tool to achieve stable gene silencing. In order to interfere with DC-SIGN function, we developed shRNA-expressing lentiviral vectors capable of conditionally suppressing DC-SIGN expression. Selectivity of inhibition of human DC-SIGN and L-SIGN and chimpanzee and rhesus macaque DC-SIGN was obtained by using distinct siRNAs. Suppression of DC-SIGN expression inhibited the attachment of the gp120 envelope glycoprotein of HIV-1 to DC-SIGN transfectants, as well as transfer of HIV-1 to target cells in trans. Furthermore, shRNA-expressing lentiviral vectors were capable of efficiently suppressing DC-SIGN expression in primary human DCs. DC-SIGN-negative DCs were unable to enhance transfer of HIV-1 infectivity to T cells in trans, demonstrating an essential role for the DC-SIGN receptor in transferring infectious viral particles from DCs to T cells. The present system should have broad applications for studying the function of DC-SIGN in the pathogenesis of HIV as well as other pathogens also recognized by this receptor.
Figures



References
-
- Abbas-Terki, T., W. Blanco-Bose, N. Deglon, W. Pralong, and P. Aebischer. 2002. Lentiviral-mediated RNA interference. Hum. Gene Ther. 13:2197-2201. - PubMed
-
- An, D. S., Y. Xie, S. H. Mao, K. Morizono, S. K. Kung, and I. S. Chen. 2003. Efficient lentiviral vectors for short hairpin RNA delivery into human cells. Hum. Gene Ther. 14:1207-1212. - PubMed
-
- Arrighi, J. F., C. Hauser, B. Chapuis, R. H. Zubler, and V. Kindler. 1999. Long-term culture of human CD34(+) progenitors with FLT3-ligand, thrombopoietin, and stem cell factor induces extensive amplification of a CD34(−)CD14(−) and a CD34(−)CD14(+) dendritic cell precursor. Blood 93:2244-2252. - PubMed
-
- Arrighi, J. F., C. Soulas, C. Hauser, S. Saeland, B. Chapuis, R. H. Zubler, and V. Kindler. 2003. TNF-alpha induces the generation of Langerin/(CD207)+ immature Langerhans-type dendritic cells from both CD14-CD1a and CD14+CD1a− precursors derived from CD34+ cord blood cells. Eur. J. Immunol. 33:2053-2063. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

