Human immunodeficiency virus type 2 Gag interacts specifically with PRP4, a serine-threonine kinase, and inhibits phosphorylation of splicing factor SF2
- PMID: 15452250
- PMCID: PMC521795
- DOI: 10.1128/JVI.78.20.11303-11312.2004
Human immunodeficiency virus type 2 Gag interacts specifically with PRP4, a serine-threonine kinase, and inhibits phosphorylation of splicing factor SF2
Abstract
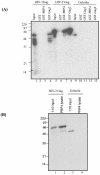
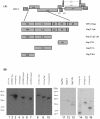
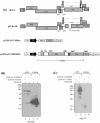
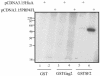
Using a yeast two-hybrid screen of a T-cell cDNA library to identify cellular proteins that bind to the human immunodeficiency virus type 2 (HIV-2) Gag polyprotein, we identified PRP4, a serine-threonine protein kinase. Specific interaction of PRP4 and HIV-2 Gag was confirmed in in vitro and in vivo assays. The interacting region of HIV-2 Gag is located in the conserved matrix and capsid domains, while both the RS (arginine-serine-rich) domain and the KS (kinase) domain of PRP4 are able to bind to HIV-2 Gag. PRP4 is not incorporated into virus particles. HIV-2 Gag is able to inhibit PRP4-mediated phosphorylation of the splicing factor SF2. This is also observed with Gag from simian immunodeficiency virus, a closely related virus, but not with Gag from human T-cell lymphotropic virus type 1. Our results provide evidence for a novel interaction between Gag and a cellular protein kinase involved in the control of constitutive splicing in two closely related retroviruses. We hypothesize that as Gag accumulates in the cell, down regulation of splicing occurs through reduced phosphorylation of SF2. At late stages of infection, this interaction may replace the function of the early viral regulatory protein Rev.
Figures


References
-
- Bevec, D., H. Jaksche, M. Oft, T. Wohl, M. Himmelspach, A. Pacher, M. Schebesta, K. Koettnitz, M. Dobrovnik, R. Csonga, F. Lottspeich, and J. Hauber. 1996. Inhibition of HIV-1 replication in lymphocytes by mutants of the Rev cofactor eIF-5A. Science 271:1858-1860. - PubMed
-
- Bukrinsky, M. I., N. Sharova, T. L. McDonald, T. Pushkarskaya, W. G. Tarpley, and M. Stevenson. 1993. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc. Natl. Acad. Sci. USA 90:6125-6129. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

