Protective efficacy of a DNA influenza virus vaccine is markedly increased by the coadministration of a Schiff base-forming drug
- PMID: 15452252
- PMCID: PMC521803
- DOI: 10.1128/JVI.78.20.11321-11326.2004
Protective efficacy of a DNA influenza virus vaccine is markedly increased by the coadministration of a Schiff base-forming drug
Abstract
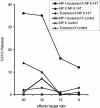
Effective vaccination against heterologous influenza virus infection remains elusive. Immunization with plasmid DNA (pDNA) expressing conserved genes from influenza virus is a promising approach to achieve cross-variant protection. However, despite having been described for more than a decade, pDNA vaccination still requires further optimization to be applied clinically as a standard vaccination approach. We have recently described a simple and efficient approach to enhance pDNA immunization, based on the use of tucaresol, a Schiff base-forming drug. In this report we have tested the ability of this drug to increase the protection conferred by pDNA vaccination against influenza virus infection. Our results demonstrate that a significant protection was achieved in two strains of mice by using the combination of pDNA and tucaresol. This protection was associated with an elevated humoral and cellular response and a switch in the type of the T helper cell (Th) immune response from type 2 to type 1. This vaccine combination represents a promising strategy for designing a clinical study for the protection from influenza and similar infections.
Figures


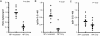
Similar articles
-
Marked enhancement of the antigen-specific immune response by combining plasmid DNA-based immunization with a Schiff base-forming drug.Infect Immun. 2002 Dec;70(12):6652-7. doi: 10.1128/IAI.70.12.6652-6657.2002. Infect Immun. 2002. PMID: 12438338 Free PMC article.
-
Mechanism of protection against influenza A virus by DNA vaccine encoding the hemagglutinin gene.Intervirology. 2000;43(4-6):322-30. doi: 10.1159/000054000. Intervirology. 2000. PMID: 11251388
-
Plasmid DNA encoding influenza virus haemagglutinin induces Th1 cells and protection against respiratory infection despite its limited ability to generate antibody responses.J Gen Virol. 2000 Jul;81(Pt 7):1737-45. doi: 10.1099/0022-1317-81-7-1737. J Gen Virol. 2000. PMID: 10859379
-
Identification of effective constituents of influenza vaccine by immunization with plasmid DNAs encoding viral proteins.Jpn J Infect Dis. 2000 Dec;53(6):219-28. Jpn J Infect Dis. 2000. PMID: 11227019 Review.
-
Schiff base forming drugs: mechanisms of immune potentiation and therapeutic potential.J Mol Med (Berl). 1996 Sep;74(9):497-504. doi: 10.1007/BF00204975. J Mol Med (Berl). 1996. PMID: 8892054 Review.
Cited by
-
Cu(II), Ni(II), and Zn(II) Complexes of Salan-Type Ligand Containing Ester Groups: Synthesis, Characterization, Electrochemical Properties, and In Vitro Biological Activities.Bioinorg Chem Appl. 2013;2013:439848. doi: 10.1155/2013/439848. Epub 2013 Jul 25. Bioinorg Chem Appl. 2013. PMID: 23983672 Free PMC article.
-
Endocine™, N3OA and N3OASq; three mucosal adjuvants that enhance the immune response to nasal influenza vaccination.PLoS One. 2013 Aug 8;8(8):e70527. doi: 10.1371/journal.pone.0070527. eCollection 2013. PLoS One. 2013. PMID: 23950951 Free PMC article.
References
-
- Abbas, A. K., A. H. Lichtman, and J. S. Pober. 2000. Cellular and molecular immunology, 4th ed. W. B. Saunders, New York, N.Y.
-
- Calarota, S., G. Bratt, S. Nordlund, J. Hinkula, A. C. Leandersson, E. Sandstrom, and B. Wahren. 1998. Cellular cytotoxic response induced by DNA vaccination in HIV-1-infected patients. Lancet 351:1320-1325. - PubMed
-
- Charo, J., A. M. Ciupitu, A. Le Chevalier De Preville, P. Trivedi, G. Klein, J. Hinkula, and R. Kiessling. 1999. A long-term memory obtained by genetic immunization results in full protection from a mammary adenocarcinoma expressing an EBV gene. J. Immunol. 163:5913-5919. - PubMed
-
- Charo, J., A. Geluk, M. Sundback, B. Mirzai, A. D. Diehl, K. J. Malmberg, A. Achour, S. Huriguchi, K. E. van Meijgaarden, J. W. Drijfhout, N. Beekman, P. van Veelen, F. Ossendorp, T. H. Ottenhoff, and R. Kiessling. 2001. The identification of a common pathogen-specific HLA class I A*0201-restricted cytotoxic T cell epitope encoded within the heat shock protein 65. Eur. J. Immunol. 31:3602-3611. - PubMed
-
- Charo, J., M. Sundback, A. Geluk, T. Ottenhoff, and R. Kiessling. 2001. DNA immunization of HLA transgenic mice with a plasmid expressing mycobacterial heat shock protein 65 results in HLA class I- and II-restricted T cell responses that can be augmented by cytokines. Hum. Gene Ther. 12:1797-1804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

