Heterologous RNA encapsidated in Pariacoto virus-like particles forms a dodecahedral cage similar to genomic RNA in wild-type virions
- PMID: 15452258
- PMCID: PMC521806
- DOI: 10.1128/JVI.78.20.11371-11378.2004
Heterologous RNA encapsidated in Pariacoto virus-like particles forms a dodecahedral cage similar to genomic RNA in wild-type virions
Abstract
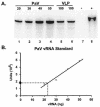
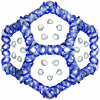
The genome of some icosahedral RNA viruses plays an essential role in capsid assembly and structure. In T=3 particles of the nodavirus Pariacoto virus (PaV), a remarkable 35% of the single-stranded RNA genome is icosahedrally ordered. This ordered RNA can be visualized at high resolution by X-ray crystallography as a dodecahedral cage consisting of 30 24-nucleotide A-form RNA duplex segments that each underlie a twofold icosahedral axis of the virus particle and interact extensively with the basic N-terminal region of 60 subunits of the capsid protein. To examine whether the PaV genome is a specific determinant of the RNA structure, we produced virus-like particles (VLPs) by expressing the wild-type capsid protein open reading frame from a recombinant baculovirus. VLPs produced by this system encapsidated similar total amounts of RNA as authentic virus particles, but only about 6% of this RNA was PaV specific, the rest being of cellular or baculovirus origin. Examination of the VLPs by electron cryomicroscopy and image reconstruction at 15.4-A resolution showed that the encapsidated RNA formed a dodecahedral cage similar to that of wild-type particles. These results demonstrate that the specific nucleotide sequence of the PaV genome is not required to form the dodecahedral cage of ordered RNA.
Figures
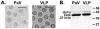



Similar articles
-
Nodavirus coat protein imposes dodecahedral RNA structure independent of nucleotide sequence and length.J Virol. 2004 Mar;78(6):2897-905. doi: 10.1128/jvi.78.6.2897-2905.2004. J Virol. 2004. PMID: 14990708 Free PMC article.
-
The structure of pariacoto virus reveals a dodecahedral cage of duplex RNA.Nat Struct Biol. 2001 Jan;8(1):77-83. doi: 10.1038/83089. Nat Struct Biol. 2001. PMID: 11135676
-
Virions of Pariacoto virus contain a minor protein translated from the second AUG codon of the capsid protein open reading frame.J Gen Virol. 2003 Oct;84(Pt 10):2847-2852. doi: 10.1099/vir.0.19419-0. J Gen Virol. 2003. PMID: 13679619
-
The structural and functional role of RNA in icosahedral virus assembly.Annu Rev Microbiol. 2006;60:51-67. doi: 10.1146/annurev.micro.60.080805.142304. Annu Rev Microbiol. 2006. PMID: 16704342 Review.
-
How and why RNA genomes are (partially) ordered in viral capsids.Curr Opin Virol. 2022 Feb;52:203-210. doi: 10.1016/j.coviro.2021.11.014. Epub 2021 Dec 24. Curr Opin Virol. 2022. PMID: 34959081 Review.
Cited by
-
Mechanical and assembly units of viral capsids identified via quasi-rigid domain decomposition.PLoS Comput Biol. 2013;9(11):e1003331. doi: 10.1371/journal.pcbi.1003331. Epub 2013 Nov 14. PLoS Comput Biol. 2013. PMID: 24244139 Free PMC article.
-
Sequence-specific, RNA-protein interactions overcome electrostatic barriers preventing assembly of satellite tobacco necrosis virus coat protein.J Mol Biol. 2013 Mar 25;425(6):1050-64. doi: 10.1016/j.jmb.2013.01.004. Epub 2013 Jan 11. J Mol Biol. 2013. PMID: 23318955 Free PMC article.
-
Bipartite viral RNA genome heterodimerization influences genome packaging and virion thermostability.J Virol. 2024 Mar 19;98(3):e0182023. doi: 10.1128/jvi.01820-23. Epub 2024 Feb 8. J Virol. 2024. PMID: 38329331 Free PMC article.
-
Infectious myonecrosis virus has a totivirus-like, 120-subunit capsid, but with fiber complexes at the fivefold axes.Proc Natl Acad Sci U S A. 2008 Nov 11;105(45):17526-31. doi: 10.1073/pnas.0806724105. Epub 2008 Nov 3. Proc Natl Acad Sci U S A. 2008. PMID: 18981418 Free PMC article.
-
Mapping RNA-capsid interactions and RNA secondary structure within virus particles using next-generation sequencing.Nucleic Acids Res. 2020 Jan 24;48(2):e12. doi: 10.1093/nar/gkz1124. Nucleic Acids Res. 2020. PMID: 31799606 Free PMC article.
References
-
- Bothner, B., A. Schneemann, D. Marshall, V. Reddy, J. E. Johnson, and G. Siuzdak. 1999. Crystallographically identical virus capsids display different properties in solution. Nat. Struct. Biol. 6:114-116. - PubMed
-
- Buchholz, U. J., S. Finke, and K. K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. - PMC - PubMed
-
- Chang, M. J., J. Kuzio, and G. W. Blissard. 1999. Modulation of translational efficiency by contextual nucleotides flanking a baculovirus initiator AUG codon. Virology 259:369-383. - PubMed
-
- Fisher, A. J., and J. E. Johnson. 1993. Ordered duplex RNA controls capsid architecture in an icosahedral animal virus. Nature (London) 361:176-182. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

