Systematic investigation of protein phase behavior with a microfluidic formulator
- PMID: 15452343
- PMCID: PMC521965
- DOI: 10.1073/pnas.0405847101
Systematic investigation of protein phase behavior with a microfluidic formulator
Abstract
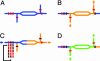
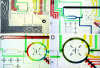
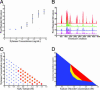
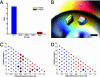
We demonstrated a microfluidic device for rapidly generating complex mixtures of 32 stock reagents in a 5-nl reactor. This "formulation chip" is fully automated and allows thousands of experiments to be performed in a single day with minimal reagent consumption. It was applied to systematically study the phase behavior of the protein xylanase over a large and complex chemical space. For each chemical formulation that demonstrated a pronounced effect on solubility, the protein phase behavior was completely mapped in the chip, generating a set of empirical phase diagrams. This ab initio phase information was used to devise a rational crystallization screen that resulted in 72-fold improvement in successful crystallization hits compared with conventional sparse matrix screens. This formulations tool allows a physics-based approach to protein crystallization that may prove useful in structural genomics efforts.
Figures

References
-
- Kendrew, J. C., Bodo, G., Dintzis, H. M., Parrish, R. G., Wyckoff, H. & Phillips, D. C. (1958) Nature 181, 662-666. - PubMed
-
- Loll, P. J. (2003) J. Struct. Biol. 142, 144-153. - PubMed
-
- Chayen, N. E. & Saridakis, E. (2002) Acta Crystallogr. D 58, 921-927. - PubMed
-
- Luft, J. R. & DeTitta, G. (1997) Macromol. Crystallogr. A. 276, 110-131. - PubMed
-
- Rosenbaum, D. F. & Zukoski, C. F. (1996) J. Cryst. Growth 169, 752-758.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

