Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen
- PMID: 15452345
- PMCID: PMC521999
- DOI: 10.1073/pnas.0405743101
Correlation between tumor regression and T cell responses in melanoma patients vaccinated with a MAGE antigen
Abstract
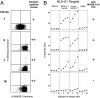
The cancer-germline gene MAGE-3 codes for tumor-specific antigens recognized on many tumors by T lymphocytes. A MAGE-3 antigen presented by HLA-A1 has been used in several vaccination trials on metastatic melanoma patients. Only a small minority of patients have shown evidence of tumor regression. Attempts to correlate the tumor rejections with the cytotoxic T lymphocyte (CTL) response against the vaccine have been hampered by the low level of these responses. In noncancerous individuals, the frequency of the T cell precursors against antigen MAGE-3.A1 is approximately 4 x 10(-7) CD8 T cells. The diversity of the T cell receptor repertoire of these anti-MAGE-3.A1 precursors was analyzed in one individual. The results indicate that it is very likely that the repertoire comprises >100 clonotypes. On this basis, it is possible to use not only the frequency of CTL precursors in the blood but also the presence of dominant clonotypes to ascertain in patients the existence of anti-MAGE-3.A1 responses as low as 10(-6) of CD8. With this approach, we observed a correlation between tumor regression and anti-MAGE-3.A1 CTL responses in patients vaccinated with a recombinant virus encoding the antigen and also in patients vaccinated with peptide-pulsed dendritic cells. In contrast, for patients showing tumor regression after vaccination with peptide alone, CTL responses were almost never observed. It is possible that even those CTL responses that are below our present detection level can trigger a sequence of events that leads to tumor regression.
Figures

References
-
- Marchand, M., van Baren, N., Weynants, P., Brichard, V., Dréno, B., Tessier, M.-H., Rankin, E., Parmiani, G., Arienti, F., Humblet, Y., et al. (1999) Int. J. Cancer 80, 219-230. - PubMed
-
- Altman, J. D., Moss, P. A. H., Goulder, P. J. R., Barouch, D. H., McHeyzer-Williams, M. G., Bell, J. I., McMichael, A. J. & Davis, M. M. (1996) Science 274, 94-96. - PubMed
-
- Genevée, C., Diu, A., Nierat, J., Caignard, A., Dietrich, P. Y., Ferradini, L., Roman-Roman, S., Triebel, F. & Hercend, T. (1992) Eur. J. Immunol. 22, 1261-1269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

