Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation
- PMID: 15452351
- PMCID: PMC521944
- DOI: 10.1073/pnas.0401346101
Restoration of an absent G1 arrest and protection from apoptosis in embryonic stem cells after ionizing radiation
Abstract
Response to DNA damage and cell-cycle regulation differ markedly between embryonic stem (ES) cells and somatic cells. ES cells require exquisitely sensitive mechanisms to maintain genomic integrity and do so, in part, by suppressing spontaneous mutation. Spontaneous mutation frequency in somatic cells is approximately 10(-4) compared with 10(-6) for ES cells. ES cells also lack a G(1) checkpoint and are hypersensitive to IR and other DNA-damaging agents. These characteristics facilitate apoptosis and the removal of cells with a mutational burden from the population, thereby keeping the population free of damaged cells. Here, we identify signaling pathways that are compromised and lead to a natural absence of aG(1) arrest in ES cells after DNA damage. The affected pathways are those mediated by p53 and p21 and by ATM, Chk2, Cdc25A, and Cdk2. In ES cells, Chk2 kinase is not intranuclear as in somatic cells but is sequestered at centrosomes and is unavailable to phosphorylate Cdc25A phosphatase and cause its degradation. Although ectopic expression of Chk2 does not rescue the p53/p21 pathway, its expression is sufficient to allow it to phosphorylate Cdc25A, activate downstream targets, restore a G(1) arrest, and protect the cell from apoptosis.
Figures
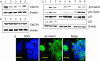


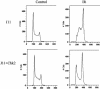

References
-
- Stambrook, P. J., Shao, C., Stockelman, M., Boivin, G., Engle, S. J. & Tischfield, J. A. (1996) Environ. Mol. Mutagen. 28, 471-482. - PubMed
-
- Aladjem, M., Spike, B. T., Rodewald, L. W., Hope, T. J., Klemm, M., Jaenisch, R. & Wahl, G. M. M. (1998) Curr. Biol. 8, 145-155. - PubMed
-
- Hirao, A., Kong, Y.-Y., Matsuoka, S., Wakeham, A., Ruland, J., Yoshida, H., Liu, D., Elledge, S. J. & Mak, T. W. (2000) Science 289, 1824-1827. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

