Electrochemical redox transformations of T1 and T2 copper sites in native Trametes hirsuta laccase at gold electrode
- PMID: 15453829
- PMCID: PMC1134750
- DOI: 10.1042/BJ20041015
Electrochemical redox transformations of T1 and T2 copper sites in native Trametes hirsuta laccase at gold electrode
Abstract
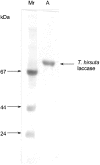
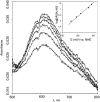
Mediatorless, electrochemically driven, redox transformations of T1 (type 1) and T2 copper sites in Trametes hirsuta laccase were studied by cyclic voltammetry and spectroelectrochemical redox titrations using bare gold electrode. DET (direct electron transfer) between the electrode and the enzyme was observed under anaerobic conditions. From analysis of experimental data it is concluded that the T2 copper site is in DET contact with gold. It was found that electron transfer between the gold surface and the T1 copper site progresses through the T2 copper site. From EPR measurements and electrochemical data it is proposed that the redox potential of the T2 site for high-potential 'blue' laccase is equal to about 400 mV versus NHE (normal hydrogen electrode) at pH 6.5. The hypothesis that the redox potentials of the T2 copper sites in low- and high-potential laccases/oxidases from totally different sources might be very similar, i.e. approx. 400 mV, is discussed.
Figures




References
-
- Gorton L., Lindgren A., Larsson T., Munteanu F. D., Ruzgas T., Gazaryan I. Direct electron transfer between heme-containing enzymes and electrodes as basis for third generation biosensors. Anal. Chim. Acta. 1999;400:91–108.
-
- Berezin I. V., Bogdanovskaya V. A., Varfolomeev S. D., Tarasevich M. R., Yaropolov A. I. Bioelectrocatalysis. Equilibrium oxygen potential in the presence of laccase. Dokl. Akad. Nauk SSSR. 1978;240:615–618.
-
- Kuznetsov B. A., Shumakovich G. P., Koroleva O. V., Yaropolov A. I. On applicability of laccase as label in the mediated and mediatorless electroimmunoassay: effect of distance on the direct electron transfer between laccase and electrode. Biosens. Bioelectron. 2001;16:73–84. - PubMed
-
- Solomon E. I., Sundaram U. M., Machonkin T. E. Multicopper oxidases and oxygenases. Chem. Rev. 1996;96:2563–2605. - PubMed
-
- Barton S. C., Kim H.-H., Binyamin G., Zhang Y., Heller A. The ‘wired’ laccase cathode: high current density electroreduction of O2 to water at +0.7 V (NHE) at pH 5. J. Am. Chem. Soc. 2001;123:5802–5803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

