Using single-particle tracking to study nuclear trafficking of viral genes
- PMID: 15454466
- PMCID: PMC1304693
- DOI: 10.1529/biophysj.104.042234
Using single-particle tracking to study nuclear trafficking of viral genes
Abstract
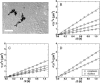
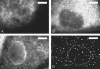
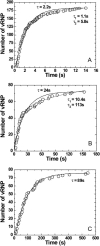
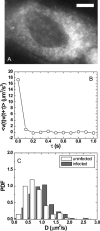
The question of how genetic materials are trafficked in and out of the cell nucleus is a problem of great importance not only for understanding viral infections but also for advancing gene-delivery technology. Here we demonstrate a physical technique that allows gene trafficking to be studied at the single-gene level by combining sensitive fluorescence microscopy with microinjection. As a model system, we investigate the nuclear import of influenza genes, in the form of ribonucleoproteins (vRNPs), by imaging single vRNPs in living cells in real time. Our single-particle trajectories show that vRNPs are transported to the nuclear envelope by diffusion. We have observed heterogeneous interactions between the vRNPs and nuclear pore complexes with dissociation rate constants spanning two orders of magnitude. Our single-particle tracking experiments also provided new insights into the regulation mechanisms for the nuclear import of vRNPs: the influenza M1 protein, a regulatory protein for the import process, downregulates the nuclear import of vRNPs by inhibiting the interactions between vRNPs and nuclear pore complexes but has no significant effect on the transport properties of vRNPs. We expect this single-particle tracking approach to find broad application in investigations of genetic trafficking.
Copyright 2004 Biophysical Society
Figures


References
-
- Bullido, R., P. Gomez-Puertas, C. Albo, and A. Portela. 2000. Several protein regions contribute to determine the nuclear and cytoplasmic localization of the influenza A virus nucleoprotein. J. Gen. Virol. 81:135–142. - PubMed
-
- Byassee, T. A., W. C. Chan, and S. Nie. 2000. Probing single molecules in living cells. Anal. Chem. 72:5606–5611. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

