Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors
- PMID: 15456405
- PMCID: PMC1134710
- DOI: 10.1042/BJ20041140
Identification and characterization of pleckstrin-homology-domain-dependent and isoenzyme-specific Akt inhibitors
Abstract
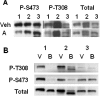
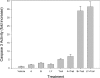
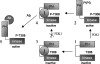
We developed a high-throughput HTRF (homogeneous time-resolved fluorescence) assay for Akt kinase activity and screened approx. 270000 compounds for their ability to inhibit the three isoforms of Akt. Two Akt inhibitors were identified that exhibited isoenzyme specificity. The first compound (Akt-I-1) inhibited only Akt1 (IC50 4.6 microM) while the second compound (Akt-I-1,2) inhibited both Akt1 and Akt2 with IC50 values of 2.7 and 21 microM respectively. Neither compound inhibited Akt3 nor mutants lacking the PH (pleckstrin homology) domain at concentrations up to 250 microM. These compounds were reversible inhibitors, and exhibited a linear mixed-type inhibition against ATP and peptide substrate. In addition to inhibiting kinase activity of individual Akt isoforms, both inhibitors blocked the phosphorylation and activation of the corresponding Akt isoforms by PDK1 (phosphoinositide-dependent kinase 1). A model is proposed in which these inhibitors bind to a site formed only in the presence of the PH domain. Binding of the inhibitor is postulated to promote the formation of an inactive conformation. In support of this model, antibodies to the Akt PH domain or hinge region blocked the inhibition of Akt by Akt-I-1 and Akt-I-1,2. These inhibitors were found to be cell-active and to block phosphorylation of Akt at Thr308 and Ser473, reduce the levels of active Akt in cells, block the phosphorylation of known Akt substrates and promote TRAIL (tumour-necrosis-factor-related apoptosis-inducing ligand)-induced apoptosis in LNCap prostate cancer cells.
Figures
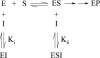

References
-
- Mitsiades C. S., Mitsiades N., Koutsilieris M., Nicholson K. M., Anderson N. G., Neri L. M., Borgatti P., Capitani S., Martelli A. M., Brazil D. P., Hemmings B. A. The Akt pathway: molecular targets for anti-cancer drug development. Curr. Cancer Drug Targets. 2004;4:235–256. - PubMed
-
- Vivanco I., Sawyers C. L. The phosphatidylinositol 3-kinase-Akt pathway in human cancer. Nat. Rev. 2002;2:489–501. - PubMed
-
- Brazil D. P., Hemmings B. A. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem. Sci. 2001;26:657–664. - PubMed
-
- Simpson L., Parsons R. PTEN: Life as a tumor suppressor. Exp. Cell Res. 2001;264:29–41. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

