Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA): design and rationale of a double-blind, placebo-controlled randomised multicenter trial [ISRCTN38327949]
- PMID: 15456517
- PMCID: PMC526218
- DOI: 10.1186/1471-2482-4-12
Probiotic prophylaxis in patients with predicted severe acute pancreatitis (PROPATRIA): design and rationale of a double-blind, placebo-controlled randomised multicenter trial [ISRCTN38327949]
Abstract
Background: Infectious complications are the major cause of death in acute pancreatitis. Small bowel bacterial overgrowth and subsequent bacterial translocation are held responsible for the vast majority of these infections. Goal of this study is to determine whether selected probiotics are capable of preventing infectious complications without the disadvantages of antibiotic prophylaxis; antibiotic resistance and fungal overgrowth.
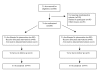
Methods/design: PROPATRIA is a double-blind, placebo-controlled randomised multicenter trial in which 200 patients will be randomly allocated to a multispecies probiotic preparation (Ecologic 641) or placebo. The study is performed in all 8 Dutch University Hospitals and 7 non-University hospitals. The study-product is administered twice daily through a nasojejunal tube for 28 days or until discharge. Patients eligible for randomisation are adult patients with a first onset of predicted severe acute pancreatitis: Imrie criteria 3 or more, CRP 150 mg/L or more, APACHE II score 8 or more. Exclusion criteria are post-ERCP pancreatitis, malignancy, infection/sepsis caused by a second disease, intra-operative diagnosis of pancreatitis and use of probiotics during the study. Administration of the study product is started within 72 hours after onset of abdominal pain. The primary endpoint is the total number of infectious complications. Secondary endpoints are mortality, necrosectomy, antibiotic resistance, hospital stay and adverse events. To demonstrate that probiotic prophylaxis reduces the proportion of patients with infectious complications from 50% to 30%, with alpha 0,05 and power 80%, a total sample size of 200 patients was calculated.
Conclusion: The PROPATRIA study is aimed to show a reduction in infectious complications due to early enteral use of multispecies probiotics in severe acute pancreatitis.
References
-
- Beger HG, Bittner R, Block S, Buchler M. Bacterial contamination of pancreatic necrosis. A prospective clinical study. Gastroenterology. 1986;91:433–8. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous