Epidermal growth factor receptor-deficient mice have delayed primary endochondral ossification because of defective osteoclast recruitment
- PMID: 15456762
- PMCID: PMC2779713
- DOI: 10.1074/jbc.M403114200
Epidermal growth factor receptor-deficient mice have delayed primary endochondral ossification because of defective osteoclast recruitment
Abstract
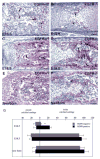
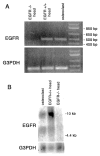
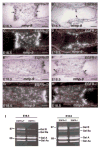
The epidermal growth factor receptor (EGFR) and its ligands function in diverse cellular functions including cell proliferation, differentiation, motility, and survival. EGFR signaling is important for the development of many tissues, including skin, lungs, intestines, and the craniofacial skeleton. We have now determined the role of EGFR signaling in endochondral ossification. We analyzed long bone development in EGFR-deficient mice. EGFR deficiency caused delayed primary ossification of the cartilage anlage and delayed osteoclast and osteoblast recruitment. Ossification of the growth plates was also abnormal resulting in an expanded area of growth plate hypertrophic cartilage and few bony trabeculae. The delayed osteoclast recruitment was not because of inadequate expression of matrix metalloproteinases, including matrix metalloproteinase-9, which have previously been shown to be important for osteoclast recruitment. EGFR was expressed by osteoclasts, suggesting that EGFR ligands may act directly to affect the formation and/or function of these cells. EGFR signaling regulated osteoclast formation. Inhibition of EGFR tyrosine kinase activity decreased the generation of osteoclasts from cultured bone marrow cells.
Figures





References
-
- Caplan AI. Ciba Found Symp. 1988;136:3021. - PubMed
-
- Olsen BR, Reginato AM, Wang W. Annu Rev Cell Dev Biol. 2000;16:191–220. - PubMed
-
- Karsenty G, Wagner EF. Dev Cell. 2002;2:389–406. - PubMed
-
- Hackel PO, Zwick E, Prenzel N, Ullrich A. Curr Opin Cell Biol. 1999;11:184–189. - PubMed
-
- Bogdan S, Klambt C. Curr Biol. 2001;11:R292–R295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

