Regulation of calcium/calmodulin-dependent protein kinase II activation by intramolecular and intermolecular interactions
- PMID: 15456810
- PMCID: PMC6729912
- DOI: 10.1523/JNEUROSCI.3604-04.2004
Regulation of calcium/calmodulin-dependent protein kinase II activation by intramolecular and intermolecular interactions
Figures
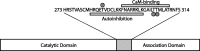

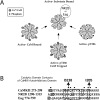
References
-
- Baines AJ (1996) Caenorhabditis elegans LIN-2A and mammalian neuronal CASK are prototypical members of a subfamily of MAGUKs (membrane-associated guanylate kinases) characterized by a common kinase-like domain and a guanylate kinase domain predicted to bind ATP. Biochem J 320: 694-696. - PMC - PubMed
-
- Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H (2001) Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411: 801-805. - PubMed
-
- Colbran RJ (1993) Inactivation of Ca2+/calmodulin-dependent protein kinase II by basal autophosphorylation. J Biol Chem 268: 7163-7170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
