Visual response properties in the dorsal lateral geniculate nucleus of mice lacking the beta2 subunit of the nicotinic acetylcholine receptor
- PMID: 15456819
- PMCID: PMC6729905
- DOI: 10.1523/JNEUROSCI.1527-04.2004
Visual response properties in the dorsal lateral geniculate nucleus of mice lacking the beta2 subunit of the nicotinic acetylcholine receptor
Abstract
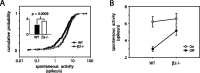
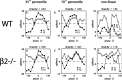
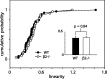
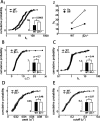
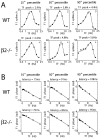
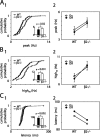
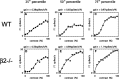
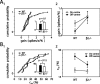
We present a quantitative description of single-cell visual response properties in the dorsal lateral geniculate nucleus (dLGN) of anesthetized adult mice lacking the beta2 subunit of the nicotinic acetylcholine receptor (beta2-/-) and compare these response properties with data from wild-type animals. Some response features, including all spatial receptive field characteristics and bursting behavior, are entirely normal in beta2-/- dLGN cells. In other respects, the responses of beta2-/- dLGN cells are quantitatively abnormal: the mutation is associated with higher spontaneous and visually evoked firing rates, faster visual response latencies, a preference for higher temporal frequencies, and a trend toward greater contrast sensitivity. The normal response properties in the beta2-/- dLGN show that none of the many effects of the mutation, including disrupted geniculate functional organization and abnormal cholinergic transmission, have any effect on spatial response characteristics and bursting behavior in dLGN neurons. The abnormal response characteristics in the beta2-/- dLGN are most interesting in that they are no worse than normal; any visual processing deficits found in studies of the beta2-/- visual cortex must therefore arise solely from abnormalities in cortical processing.
Figures

Similar articles
-
Abnormal functional organization in the dorsal lateral geniculate nucleus of mice lacking the beta 2 subunit of the nicotinic acetylcholine receptor.Neuron. 2003 Dec 18;40(6):1161-72. doi: 10.1016/s0896-6273(03)00789-x. Neuron. 2003. PMID: 14687550
-
A Comparison of Visual Response Properties in the Lateral Geniculate Nucleus and Primary Visual Cortex of Awake and Anesthetized Mice.J Neurosci. 2016 Nov 30;36(48):12144-12156. doi: 10.1523/JNEUROSCI.1741-16.2016. J Neurosci. 2016. PMID: 27903724 Free PMC article.
-
Biochemical and anatomical subdivision of the dorsal lateral geniculate nucleus in normal mice and in mice lacking the beta2 subunit of the nicotinic acetylcholine receptor.Vision Res. 2004 Dec;44(28):3365-76. doi: 10.1016/j.visres.2004.09.003. Vision Res. 2004. PMID: 15536004
-
The influence of the corticothalamic projection on responses in thalamus and cortex.Philos Trans R Soc Lond B Biol Sci. 2002 Dec 29;357(1428):1823-34. doi: 10.1098/rstb.2002.1159. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12626015 Free PMC article. Review.
-
The control of retinogeniculate transmission in the mammalian lateral geniculate nucleus.Exp Brain Res. 1986;63(1):1-20. doi: 10.1007/BF00235642. Exp Brain Res. 1986. PMID: 3015651 Review.
Cited by
-
Developmental homeostasis of mouse retinocollicular synapses.J Neurosci. 2007 Feb 14;27(7):1746-55. doi: 10.1523/JNEUROSCI.4383-06.2007. J Neurosci. 2007. PMID: 17301182 Free PMC article.
-
Retinal waves are unlikely to instruct the formation of eye-specific retinogeniculate projections.Neural Dev. 2009 Jul 6;4:25. doi: 10.1186/1749-8104-4-25. Neural Dev. 2009. PMID: 19580684 Free PMC article. Review.
-
Visual Information Processing in the Ventral Division of the Mouse Lateral Geniculate Nucleus of the Thalamus.J Neurosci. 2020 Jun 24;40(26):5019-5032. doi: 10.1523/JNEUROSCI.2602-19.2020. Epub 2020 Apr 29. J Neurosci. 2020. PMID: 32350041 Free PMC article.
-
Disruption of Cholinergic Retinal Waves Alters Visual Cortex Development and Function.bioRxiv [Preprint]. 2024 Apr 15:2024.04.05.588143. doi: 10.1101/2024.04.05.588143. bioRxiv. 2024. PMID: 38644996 Free PMC article. Preprint.
-
Formation of eye-specific retinogeniculate projections occurs prior to the innervation of the dorsal lateral geniculate nucleus by cholinergic fibers.Thalamus Relat Syst. 2005;3(2):157-163. doi: 10.1017/S1472928807000167. Thalamus Relat Syst. 2005. PMID: 19367340 Free PMC article.
References
-
- Albrecht DG, Hamilton DB (1982) Striate cortex of monkey and cat: contrast response function. J Neurophysiol 48: 217-237. - PubMed
-
- Alitto HJ, Usrey WM (2003) Corticothalamic feedback and sensory processing. Curr Opin Neurobiol 13: 440-445. - PubMed
-
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB (2000) Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming ON and OFF circuits in the inner retina. J Neurosci 20: 7672-7681. - PMC - PubMed
-
- Chen C, Regehr WG (2000) Developmental remodeling of the retinogeniculate synapse. Neuron 28: 955-966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
