Abl tyrosine kinase promotes dendrogenesis by inducing actin cytoskeletal rearrangements in cooperation with Rho family small GTPases in hippocampal neurons
- PMID: 15456825
- PMCID: PMC6729913
- DOI: 10.1523/JNEUROSCI.1264-04.2004
Abl tyrosine kinase promotes dendrogenesis by inducing actin cytoskeletal rearrangements in cooperation with Rho family small GTPases in hippocampal neurons
Abstract
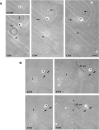
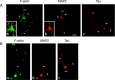
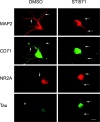
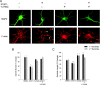
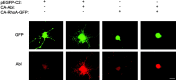
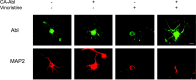
Nonreceptor tyrosine kinase Abl is an actin-binding protein and a key regulator of neuronal axonal development. Although Abl family kinases also are localized in dendrites and are implicated in postsynaptic functions, it is not clear how Abl kinases regulate dendritic morphogenesis. Using a developing hippocampal culture as a model, we found that the inhibition of Abl kinases by STI571 leads to a remarkable simplification of dendritic branching similar to the phenotype caused by an increased activity of small GTPase RhoA. Time-lapse microscopic imaging reveals a prominent reduction of dendritic branching. In contrast, neurons expressing a constitutively active v-abl construct (CA-Abl) show an exuberant microtubule-associated protein 2-positive (MAP2-positive) dendrite outgrowth, suggesting that Abl modulates dendritic growth. Biochemical assays using a glutathione S-transferase pull-down method to determine GTP-bound active Rho GTPases demonstrate that Abl inhibition increases RhoA activity but has no effect on the activity of Rac1 or Cdc42. At the cellular level the alteration of Abl also changes actin organization consistent with RhoA inhibition. Suppression of the RhoA downstream effector Rho kinase reverses STI571-induced dendritic simplification, demonstrating that activity of the Rho pathway is responsible for the Abl-induced changes in dendrogenesis. Furthermore, CA-Abl-induced neurite outgrowth is blocked by the expression of a constitutively active RhoA construct. The CA-Abl phenotype is not affected by destabilization of microtubules but is reversed partially when actin filaments are stabilized with jasplakinolide. Together, these studies support a critical role for Abl kinases in regulating dendrogenesis by inducing actin cytoskeletal rearrangements in cooperation with Rho GTPases.
Figures




References
-
- Benard V, Bohl BP, Bokoch GM (1999) Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem 274: 13198-13204. - PubMed
-
- Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S (2000) A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron 26: 431-441. - PubMed
-
- Bradke F, Dotti CG (1999) The role of local actin instability in axon formation. Science 283: 1931-1934. - PubMed
-
- Buchdunger E, Zimmermann J, Mett H, Meyer T, Muller M, Druker BJ, Lydon NB (1996) Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res 56: 100-104. - PubMed
-
- Buchdunger E, Cioffi CL, Law N, Stover D, Ohno-Jones S, Druker BJ, Lydon NB (2000) Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-Kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther 295: 139-145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
