ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks
- PMID: 15456834
- PMCID: PMC6729897
- DOI: 10.1523/JNEUROSCI.2660-04.2004
ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks
Abstract
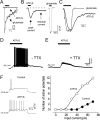
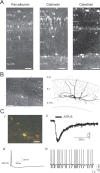
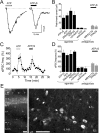
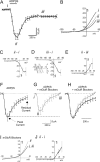
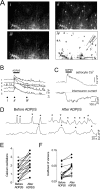
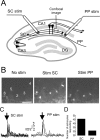
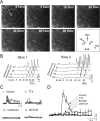
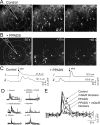
We investigated the role of extracellular ATP at astrocytes and inhibitory GABAergic interneurons in the stratum radiatum area of the mouse hippocampus. We show that exogenously applied ATP increased astrocyte intracellular Ca2+ levels and depolarized all calbindinand calretinin-positive interneurons in the stratum radiatum region of mouse hippocampus, leading to action potential firing and enhanced synaptic inhibition onto the postsynaptic targets of interneurons. Electrophysiological, pharmacological, and immunostaining studies suggested that the effect of ATP on interneurons was mediated by P2Y1 receptors, and that the depolarization of interneurons was caused by the concomitant reduction and activation of potassium and nonselective cationic conductances, respectively. Electrical stimulation of the Schaffer collaterals and perforant path, as well as local stimulation within the stratum radiatum, evoked increases in intracellular Ca2+ in astrocytes. Facilitation of GABAergic IPSCs onto interneurons also occurred during electrical stimulation. Both the stimulation-evoked increases in astrocyte Ca2+ levels and facilitation of GABAergic IPSCs were sensitive to antagonists of P2Y1 receptors and mimicked by exogenous P2Y1 receptor agonists, suggesting that endogenously released ATP can activate P2Y receptors on both astrocytes and interneurons. Overall, our data are consistent with the hypothesis that ATP released from neurons and astrocytes acts on P2Y1 receptors to excite interneurons, resulting in increased synaptic inhibition within intact hippocampal circuits.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
