Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy
- PMID: 15456836
- PMCID: PMC6729896
- DOI: 10.1523/JNEUROSCI.2877-04.2004
Altered expression of the delta subunit of the GABAA receptor in a mouse model of temporal lobe epilepsy
Abstract
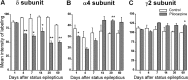
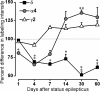
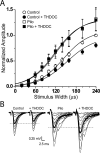
delta Subunit-containing GABA(A) receptors are located predominantly at nonsynaptic sites in the dentate gyrus where they may play important roles in controlling neuronal excitability through tonic inhibition and responses to GABA spillover. Immunohistochemical methods were used to determine whether delta subunit expression was altered after pilocarpine-induced status epilepticus in C57BL/6 mice in ways that could increase excitability of the dentate gyrus. In pilocarpine-treated animals, the normal diffuse labeling of the delta subunit in the dentate molecular layer was decreased by 4 d after status epilepticus (latent period) and remained low throughout the period of chronic seizures. In contrast, diffuse labeling of alpha4 and gamma2 subunits, potentially interrelated GABA(A) receptor subunits, was increased during the chronic period. Interestingly, delta subunit labeling of many interneurons progressively increased after pilocarpine treatment. Consistent with the observed changes in delta subunit labeling, physiological studies revealed increased excitability in the dentate gyrus of slices obtained from the pilocarpine-treated mice and demonstrated that physiological concentrations of the neurosteroid tetrahydrodeoxycorticosterone were less effective in reducing excitability in the pilocarpine-treated animals than in controls. The findings support the idea that alterations in nonsynaptic delta subunit-containing GABA(A) receptors in both principal cells and interneurons could contribute to increased seizure susceptibility in the hippocampal formation in a temporal lobe epilepsy model.
Figures






Comment in
-
GABA receptors gone bad: the wrong place at the wrong time.Epilepsy Curr. 2005 May-Jun;5(3):91-4. doi: 10.1111/j.1535-7511.2005.05304.x. Epilepsy Curr. 2005. PMID: 16145612 Free PMC article. No abstract available.
References
-
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB (2001) α4β3δ GABAA receptors characterized by FRET-derived measurements of membrane potential. J Biol Chem 276: 38934-38939. - PubMed
-
- Araujo F, Ruano D, Vitorica J (1998) Absence of association between δ and γ2 subunits in native GABAA receptors from rat brain. Eur J Pharmacol 347: 347-353. - PubMed
-
- Belelli D, Casula A, Ling A, Lambert JJ (2002) The influence of subunit composition on the interaction of neurosteroids with GABAA receptors. Neuropharmacology 43: 651-661. - PubMed
-
- Bencsits E, Ebert V, Tretter V, Sieghart W (1999) A significant part of native γ-aminobutyric acidA receptors containing α4 subunits do not contain γ or δ subunits. J Biol Chem 274: 19613-19616. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
